Chapter: Pharmaceutical Biotechnology: Fundamentals and Applications : Formulation of Biotech Products, Including Biopharmaceutical Considerations
Freezing - Excipients Used in Parenteral Formulations of biotech Products
Freezing
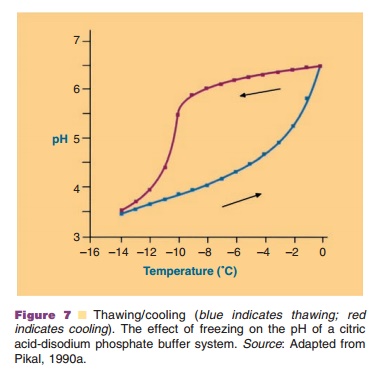
In the freezing step (Fig. 6) the temperature of the aqueous system in the vials is lowered. Ice crystal formation does not start right at the thermodynamic or equilibrium freezing point, but supercooling occurs. That means that crystallization often only occurs when temperatures of –15 C or lower have been reached. During the crystallization step the temperature may temporarily rise in the vial, because of the generation of crystallization heat. During the cooling stage, concentration of the protein and excipients occurs because of the growing ice crystal mass at the expense of the aqueous water phase. This can cause precipitation of one or more of the excipients, which may consequently result in pH shifts (see above and Fig. 7) or ionic strength changes. It may also induce protein denaturation. Cooling of the vials is done through lowering the temperature of the shelf. Selecting the proper cooling scheme for the shelf, and consequently vial, is important as it dictates the degree of supercooling and ice crystal size. Small crystals are formed during fast cooling; large crystals form at lower cooling rates. Small ice crystals are required for porous solids and fast sublimation rates (Pikal, 1990a).
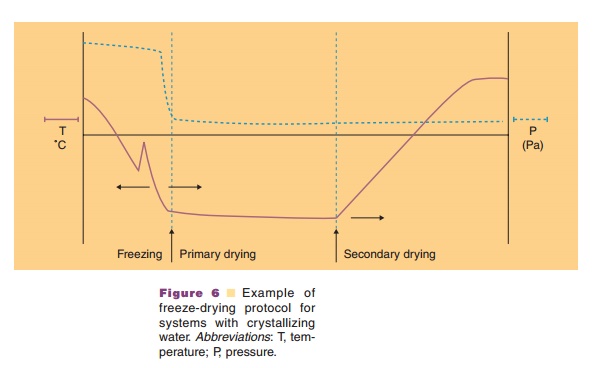
If the system does not (fully) crystallize but forms an amorphous mass upon cooling, the tem-perature in the “freezing stage” should drop below Tg, the glass transition temperature. In amorphoussystems the viscosity changes dramatically in the temperature range around the Tg: a “rubbery” state exists above and a glass state below the Tg.
At the start of the primary drying stage no “free and fluid” water should be present in the vials. Minus forty degrees Celsius is a typical freezing temperature before sublimation is initiated through pressure reduction.
Related Topics