Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Relation between enthalpy `H' and internal energy `U'
Enthalpy
In chemistry most of the chemical reactions are carried out at constant
pressure. To measure heat changes of system at constant pressure, it is useful
to define a new thermodynamic state function called Enthalpy `H'.
H is defined as sum of the internal energy `U' of a system and the
product of Pressure and Volume of the system.
That is,
H = U + PV
H = U + PV
Characteristics of H
Enthalpy, H depends on three state functions U, P, V and hence it is
also a state function. H is independent of the path by which it is reached.
Enthalpy is also known by the term `heat content'.
Relation between
enthalpy `H' and internal energy `U'
When the
system at constant pressure undergoes changes from an initial state with H1, U1,
V1, P parameters to a final state with H2, U2, V2, P parameters the chamge in
enthalpy ∆H, is given by,
∆H = (H2 - H1) =
(U2 - U1) + P(V2 - V1)
∆H = ∆U +
P∆V
Considering ∆U = q -w or q - P∆V (assuming P- V work), ∆U + P∆V becomes equal to 'qp'.
'qp' is the heat absorbed by the system at constant pressure for increasing
the volume from V1 to V2. This is so because, -w
indicates that work is done by the system. Therefore volume increase against
constant pressure is considered.
Eqn. becomes qp = ∆U + P ∆V
= ∆H
Or ∆H = qp
`qp'
is the heat absorbed by the system at constant pressure and is considered as
the heat content of the system.
Heat effects measured at constant pressure
indicate changes in enthalpy of a system and not in changes of internal energy
of the system. Using calorimeters operating at constant pressure, the enthalpy
change of a process can be measured directly.
Considering a system of gases which are
chemically reacting to produce product gases with Vr and Vp as the total
volumes of the reactant and product gases respectively, and nr and np
as the number of moles of gaseous reactants and products, then using ideal gas
law we can write that, at constant temperature and constant pressure,
PVr = nrRT and PVp = npRT.
Then considering reactants as initial state and products as final state
of the system,
P(Vp
- Vr) = RT (np - nr)
P∆V = ∆ngRT
where,
∆ng refers to the to the difference in the number of moles of product and reactant gases.
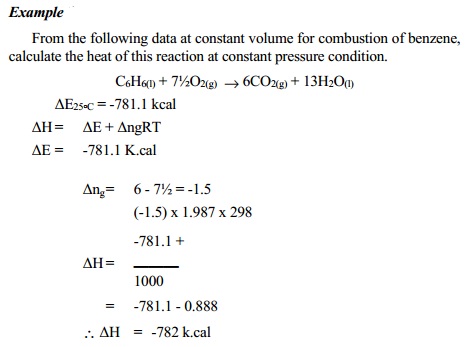
But, we already know that, ∆H = ∆U + P ∆V
∆H = ∆U + ∆ngRT
Incertain
processes internl energy change ∆U = ∆E also.
Standard enthalpy
changes
The standard enthalpy of a reaction is the enthalpy change for a
reaction when all the participating substances (elements and compounds) are
present in their standard states.
The standard state of a substance at any specified temperature is its
pure form at 1 atm pressure. For example standard state of solid iron at 500 K
is pure iron at 500 K and 1 atm. Standard conditions are denoted by adding the
superscript 0 to the symbol ∆H.
For a reaction, the standard enthalpy change is denoted by ∆rH0. Similarly, the standard enthalpy changes for combustion, formation, etc. are denoted by ∆cH0 and ∆fH0 etc respectively. Generally the reactants are presented in their standard states during the enthalpy change.
Related Topics