Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Rate equation for first order reactions
Rate equation for first order
reactions
The reactions in which, the overall rate of the
reaction is proportional to the first power of concentration of one of the
reactants only are called as first order reactions. Consider the reaction
A -- k1 -- > products
Rate of reaction = -d[A]
/ dt
= = k [A]1.0
where k1
is the rate constant of the first order reaction.
At the beginning of the reaction, time ' t' = 0, let the concentration of A be ' a' mole.lit -1 . After the
reaction has proceeded for some time 't',
let the concentration of A that has reacted be x mole.lit-1 . The concentration of unreacted A
remaining at time ' t' will be ( a - x)
mole.lit-1 . The rate of the reaction will be dx/dt. For a first
order reaction, rate = dx/dt
= k1 (a - x) ……. (2)
upon integrating, equation 2 becomes,

which is, -ln(a
- x) = k 1t + c
c = integration constant
at time, t
= 0, x = 0.
in equation 3,
- ln ( a
- 0) = k1 ´ 0 +
C or C = -ln a.
Substituting C value in equation 3
Unit of k1
is sec-1
This equation is known as the first order rate
constant equation.
This equation can be used for determining the
rate constant of a first order reaction based on the experimental data of (a) and (a - x) at different
periods of time 't'. Sometimes, the
following expression is also used.
k1 = ( 2
303 / t2-t1 ) log (a-x1 / a-x2)
where x1
and x2 are the amounts
reacted in t1 and
t2 periods of time.
Characteristics of first
order reaction
When the
concentration of the reactant is increased by 'n' times, the rate of reaction is also increased by n times. That
is, if the concentration of the reactant is doubled, the rate is doubled.
The unit of rate constant of a first order
reaction is sec-1 or time-1
.
k1 = rate /
( a - x ) = mol.lit -1 sec-1 / mol.lit -1 = sec-1
The time required to complete a definite
fraction of reaction is independent of the initial concentration, of the
reactant if t1/u is the time of one '
u'th fraction of reaction
to take place then from equation 4,
x = a / u and
t1/u = (2 303 / k1) log ( a/(a-a/u) )
t1/u = (2 303 / k1) log ( u/(u-1) )
since k1
= rate constant, t1/u is
independent of initial concentration 'a'.
Examples of first order reactions
1. All radioactive transformations follow first
order kinetics. For example,
92U238 -- -- > 90Th234 + 2He4
Decomposition of sulphuryl chloride in the gas
phase proceed by first order kinetics.
SO2Cl2(g) -- -- >
SO2(g) + Cl2(g)
Inversion
of sucrose in acidic aqueous medium follows first order reaction.
C12H22O11
+ H2O --H+ --- >
C6H12O6 (Glucose)
+ C6H12O6
(Fructose)Decomposition of nitrogen pentoxide in CCl4 medium also
exhibits first order kinetics.
N2O5 --- > 2 NO2
+ ½ O2
There are many other reactions that proceed by
first order kinetics. We shall study some of the reactions that are
experimentally followed by first order kinetic expressions including the
parameters that change with concentration of the reactants or products which
change with time of progress of the reaction.
Let us consider some of the first order
reactions in detail :
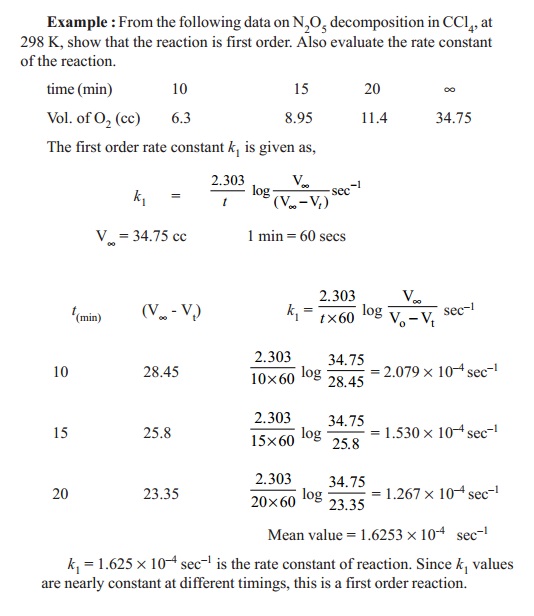
1. Decomposition of nitrogen
pentoxide in CCl4
N2O2 ---- k1
--- > 2NO2 + 1/2O2
At time t
= 0, the volume of oxygen liberated is zero. Let Vt and V¥ be the
measured volumes of oxygen liberated after the reactant has reacted in 't' time and at completion (t = ¥). Initial concentration of N2O5
is proportional to total volume of oxygen liberated (i.e.,) (V¥).
(V¥-Vt) is proportional to undecomposed
N2O5 at time ' t'.
K1 = (2.303 / t) log ( V¥ /(V¥-Vt) )sec-1
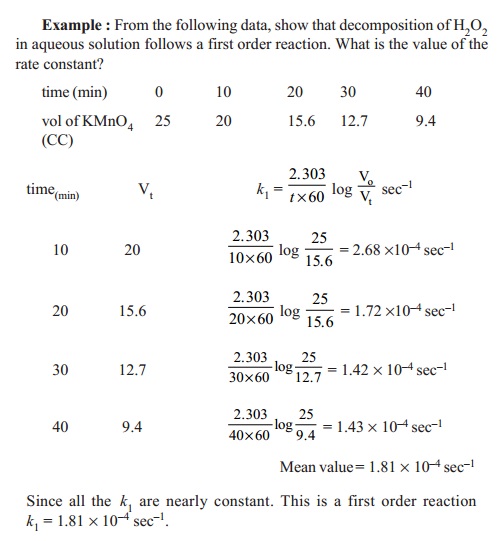
2. Decomposition of H2O2
in aqueous solution
H2O2 --- Pt
--- > H2O + ½ O2
The decomposition of H2O2
in aqueous medium in the presence of Pt catalyst follows a first order
reaction. The progress of the reaction is followed by titrating equal volumes
of the reaction mixture at regular time intervals against standard KMnO4
solution.
Since volume of KMnO4 used in the
titration is a measure of concentration of undecomposed H2O2,
volume of KMnO4 consumed at t =
0 is 'V o' which
proportional to 'a', the initial
concentration of H 2O2.
Vt is proportional to
unreacted H2O2 which is similarly (a - x ). Similarly (Vo-Vt) is proportional to
' x', the concentration of H2O2
reacted in time interval 't'. Vt
is the volume of KMnO4 consumed after time ' t' of the reaction.
The first order rate constant ' k1' of the reaction is,
K1 = (2.303 / t) log ( V0 / Vt )sec-1
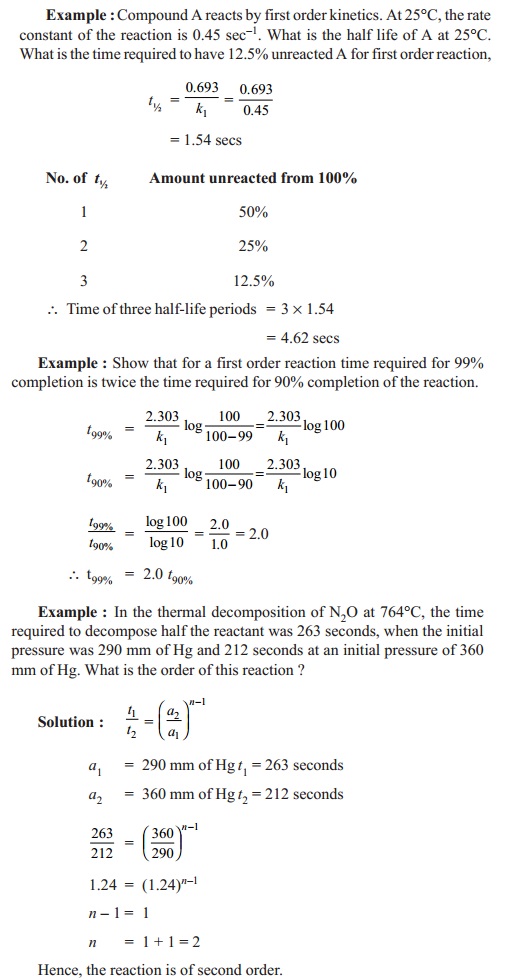
Half life period 't½'
Half life period, 't ½', of a
reaction is defined as the time required to reduce the concentration of a
reactant to one half of its initial value. t½ values are calculated
by using the integrated rate equation of any order of a reaction.
For first order reaction,
K1 = (2.303 / t) log ( a / (a-x) )sec-1
if amount reacted x = 2 then t= t1/2
t1/2 = (2.303 / k1) log ( a / (a-(a/2)) )
t1/2 = (2.303 / k1) log (2.0)
t1/2 = 0.693/ k1 secs
Thus half life period of a first order reaction
is independent of the initial concentration of the reactant and also, inversely
proportional to the rate constant of the reaction.

Related Topics