Chapter: Organic Chemistry: Alkenes and alkynes
Preparation of alkenes and alkynes
PREPARATION OF ALKENES AND ALKYNES
Key Notes
Alkenes
Alkenes
can be synthesized by the reduction of alkynes or by the elimina-tion of alkyl
halides or alcohols. Vicinal dibromides can be debrominated by treatment with
zinc dust in acetic acid or with sodium iodide in acetone.
Alkynes
Alkenes
can be treated with bromine to give a vicinal dibromide. Treatment of the dibromide
with a strong base such as sodium amide results in the loss of two molecules of
hydrogen bromide (dehydrohalogenation) and the for-mation of an alkyne.
Alkenes
Alkenes can be obtained by the transformation
of various functional groups such as the reduction of alkynes, the elimination
of alkyl halides, or the elimination of alcohols.
Alkenes can also be synthesized from vicinal
dibromides, that is, molecules which have bromine atoms on neighboring carbon
atoms. This reaction is called a debromination reaction and is carried out by
treating the dibromide with sodium iodide in acetone or with zinc dust in
acetic acid (Fig. 1).
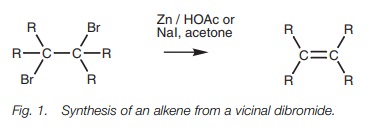
The dibromide itself is usually prepared from
the same alkene and so the reaction is not particularly useful for the synthesis
of alkenes. It is useful, however, in protection strategy. During a lengthy
synthesis, it may be necessary to protect
a double bond
so that it
does not undergo
any undesired reactions. Bromine can be added to form the
dibromide and removed later by debromina- tion in order to restore the
functional group.
Alkynes
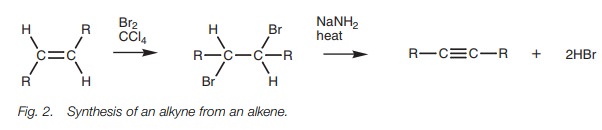
Alkynes can be synthesized from alkenes through
a two-step process which involves the electrophilic
addition of bromine to form a vicinal dibromide then dehydrohalogenation with strong base (Fig. 2). The second stage involves the loss of two molecules of
hydrogen bromide and so two equivalents of base are required.
Related Topics