Chapter: Organic Chemistry: Alkenes and alkynes
Alkylation of terminal alkynes
ALKYLATION OF TERMINAL ALKYNES
Key Notes
Terminal alkynes
A
terminal alkyne is defined as an alkyne having a hydrogen substituent.
The
hydrogen of a terminal alkyne is weakly acidic and can be removed with a strong
base such as sodium amide to produce an alkynide ion.
Alkylation
The
alkynide ion can be treated with a primary alkyl halide to produce a
disubstituted alkyne.
Terminal alkynes
A terminal alkyne is defined as an alkyne
having a hydrogen substituent (Fig. 1).
This hydrogen substituent is acidic and can be removed with strong base (e.g.
sodium amide NaNH2) to produce an alkynide (Fig. 2). This
is an example of an acid–base reaction.
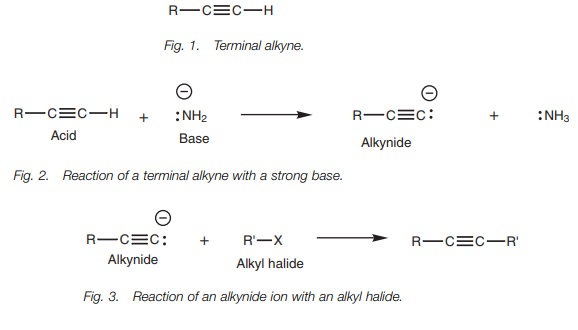
Alkylation
Once the alkynide has been formed, it can be
treated with an alkyl halide to produce more complex alkynes (Fig. 3). This
reaction is known as an alkylation as far as the alkynide is concerned, and is
an example of nucleophilic substitution as far as the alkyl halide is
concerned.
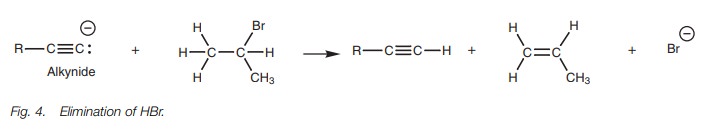
This reaction works best with primary alkyl
halides. When secondary or ter-tiary alkyl halides are used, the alkynide
reacts like a base and this results in elimination of hydrogen halide from the
alkyl halide to produce an alkene.
Related Topics