Chapter: Organic Chemistry: Alkenes and alkynes
Carbocation stabilization
CARBOCATION STABILIZATION
Key Notes
Stabilization
Carbocations are
stabilized by induction,
hyperconjugation, or
delocalization.
Inductive effects
Alkyl
groups have an electron-donating effect on any neighboring positive charge. The
more alkyl groups attached, the greater the stabilizing effect.
Hyperconjugation
In
hyperconjugation, the vacant 2p orbital of the carbocation can interact with
the σ orbitals of neighboring C–H bonds. As a result, the σ electrons ofthe C–H
bond can spend a small amount of time entering the space occu-pied by the 2p
orbital such that the latter orbital is not completely empty.
This
interaction serves to spread or delocalize the positive charge to neigh- boring
σ bonds and thus stabilize it. The more
substituents present, the more chances there are for hyperconjugation.
Stabilization
Positively charged species such as carbocations
are inherently reactive and unstable. The more unstable they are, the less
easily they are formed and the less likely the overall reaction. Any factor
which helps to stabilize the positive charge (and by inference the carbocation)
will make the reaction more likely. There are three ways in which a positive
charge can be stabilized: inductive effects, hyperconjugation, and
delocalization. We have already seen the effects of delocalization in
stabilizing the bromonium ion. We will now look at the effects of induction and
hyperconjugation.
Inductive effects
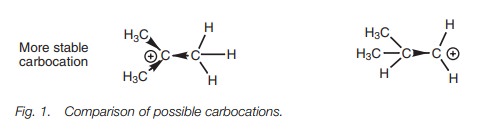
Alkyl groups can donate electrons towards a neighboring positive center and this helps to stabilize the ion since some of the positive charge is partially dispersed over the alkyl group (Fig. 1). The more alkyl groups which are attached, the greater the electron donating power and the more stable the carbocation.
Hyperconjugation
Both carbons of an alkene are sp2 hybridized. However, this
is altered on formation of the carbocation (Fig.
2). When an alkene reacts with an electrophile such as a proton, both
electrons in the π bond are used to form a new σ bond to
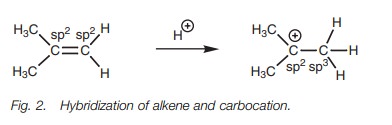
The other carbon containing the positive charge remains as an sp2center. This means that
it has three sp2
hybridized orbitals (used for the three bonds still present) and one vacant 2p orbital which is not involved in
bonding. Hyperconjugation involves the overlap of the vacant 2p orbital with a neighboring C–H σ-bond orbital (Fig. 3).
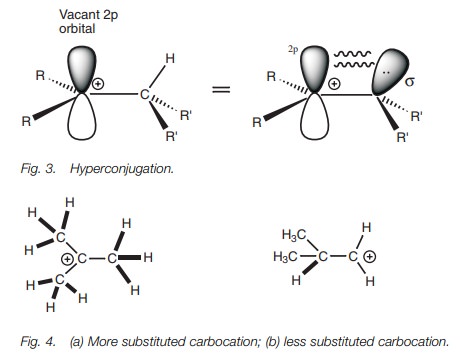
This interaction means that the 2p orbital is not completely vacant since
the σ electrons of the C–H bond can spend a small
amount of time entering the space occupied by the 2p orbital. This means that the C–H bond becomes slightly elec-tron
deficient. As a result, the positive charge is delocalized and hence
stabilized. The more alkyl groups attached to the carbocation, the more
possibilities there are for hyperconjugation and the more stable the
carbocation. For example, the more substituted carbocation (Fig. 4a) can be stabilized by
hyperconjugation to nine C–H bonds, whereas the less substituted carbocation (Fig. 4b) can only be stabilized by hyperconjugation
to one C–H bond.
Related Topics