Chapter: Organic Chemistry: Alkenes and alkynes
Electrophilic addition to symmetrical alkenes
ELECTROPHILIC ADDITION TO SYMMETRICAL ALKENES
Key Notes
Reactions
Alkenes
readily undergo electrophilic addition reactions. The π bond is
involved in the reaction and new substituents are added to either end of the
original alkene.
Symmetrical and unsymmetrical alkenes
Symmetrical
alkenes have the same substituents at each end of the double bond.
Unsymmetrical alkenes do not.
Hydrogen halide addition
Treating
an alkene with a hydrogen halide results in the formation of an alkyl halide.
The proton from the hydrogen halide adds to one end of the double bond and the
halogen atom to the other. The mechanism of elec-trophilic addition is a two
stage process which goes through a carbocation intermediate. In the first
stage, the alkene acts as a nucleophile and the hydrogen halide acts as an
electrophile. In the second stage of the mecha-nism, the halide ion acts as a
nucleophile and the carbocation intermediate is an electrophile.
Halogen addition
Alkenes
react with bromine or chlorine to produce vicinal dihalides with the halogen
atoms adding to each end of the double bond. The reaction is useful in the
protection or purification of alkenes or as a means of synthe-sizing alkynes.
The halogen molecule is polarized as it approaches the alkene double bond thus
generating the required electrophilic center. The intermediate formed is called
a bromonium ion intermediate in the case of bromine and is stabilized since it
is possible to share or delocalize the posi-tive charge between three atoms. If
the reaction is carried out in water, water can act as a nucleophile and
intercept the reaction intermediate to form a halohydrin where a halogen atom
is added to one end of the double bond and a hydroxyl group is added to the
other.
Alkenes to alcohols
Alkenes
can be converted to alcohols by treatment with aqueous acid (e.g. sulfuric
acid). Milder conditions can be used if mercuric acetate is used to produce an
organomercury intermediate which is reduced with sodium borohydride.
Alkenes to ethers
A
similar reaction to the mercuric acetate/sodium borohydride synthesis of
alcohols allows the conversion of alkenes to ethers. In this case, mercuric
trifluoracetate is used.
Alkenes to arylalkanes
Alkenes
can be reacted with aromatic rings to give arylalkanes. The reaction is known
as a Friedel–Crafts alkylation of the aromatic ring but can also be viewed as
another example of an electrophilic addition to an alkene.
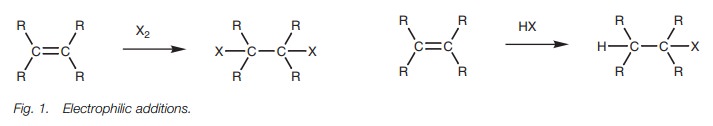
Reactions
Many of the reactions which alkenes undergo
take place by a mechanism known as electrophilic
addition (Fig. 1). In these
reactions, theπbond of the double bond has been used to form a
bond to an incoming electrophile and is no longer present in the product.
Furthermore, a new substituent has been added to each of the carbon atoms.
Symmetrical and unsymmetrical alkenes
In this section we shall look at the
electrophilic addition of symmetrical alkenes.
A symmetrical alkene is an alkene which has the
same substituents at each end of the double bond (Fig. 2a). Unsymmetrical alkenes have different substituents at each
end of the double bond (Fig. 2b).

Hydrogen halide addition
Alkenes react with hydrogen halides (HCl, HBr,
and HI) to give an alkyl halide.
The hydrogen halide molecule is split and the hydrogen atom adds to one end of the double bond while the halogen atom adds to the other. The reaction of HBr with 2,3-dimethyl-2-butene is an example of this reaction (Fig. 3). In this reaction, the alkene acts as a nucleophile. It has an electron-rich double bond containing four electrons, two of which make up a strong σ bond and two of which make up a weaker π bond.
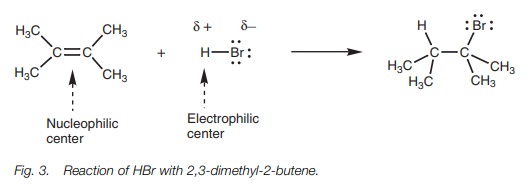
The double bond can be viewed as a nucleophilic center.
Hydro-gen bromide has a polar H–Br bond and so the hydrogen is an electrophilic
center and the bromine is a nucleophilic center. However, halogen atoms are
extremely weak nucleophilic centers and so this molecule is more likely to
react as an electrophile through its electrophilic hydrogen.
In the first step of electrophilic addition (Fig. 4), the alkene acts as a
nucleophile and uses its two π electrons to form a new bond to the hydrogen
of HBr. As this new bond is formed, the H–Br bond breaks since hydrogen is only
allowed one bond. Both electrons in that bond end up on the bromine atom to
produce a bro-mide ion. Since the electrons from the π bond have been used for the formation of a new σ bond, the π bond is no longer present. As a result, the
‘left hand’ carbon has been left with only three bonds and becomes positively
charged. This is known as a carbocation
since the positive charge is on a carbon atom.
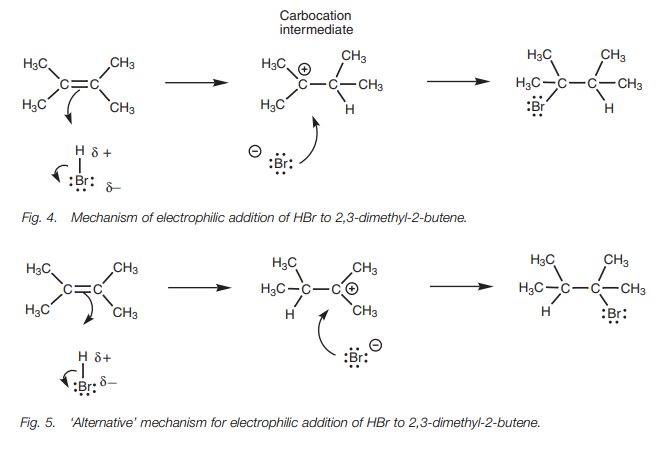
This structure is known as a reaction intermediate. It is a reactive
species and will not survive very long with the bromide ion in the vicinity.
The carbocation is an electrophile since it is positively charged. The bromide
ion is a nucleophile since it is negatively charged. Therefore, the bromide ion
uses one of its lone pairs of electrons to form a new σ bond to the carbocation and the final product is formed.
The mechanism involves the addition of HBr to
the alkene. It is an electrophilic addition since the first step of the
mechanism involves the addition of the elec-trophilic hydrogen to the alkene. Note
that the second step involves a nucleophilic addition of the bromide ion to the
carbocation intermediate, but it is the first step which defines this reaction.
In the mechanism shown (Fig. 4), the π electrons of the alkene provide the elec-trons for a new bond between the right hand carbon and hydrogen. They could equally well have been used to form a bond between the left hand carbon and hydrogen (Fig. 5).
With a symmetrical alkene, the product is the
same and so it does not matter which end of the double bond is used for the new
bond to hydrogen. The chances are equal of the hydrogen adding to one side or
the other.
The electrophilic additions of H–Cl and H–I
follow the same mechanism to pro-duce alkyl chlorides and alkyl iodides
respectively.
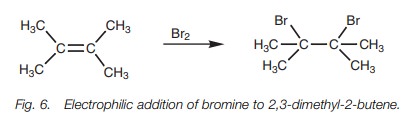
Halogen addition
The reaction of an alkene with a halogen such as bromine or chlorine results in the formation of a vicinal dihalide. The halogen molecule is split and the halogens are added to each end of the double bond (Fig. 6). Vicinal dibromides are useful in the purification or protection of alkenes since the bromine atoms can be removed under different reaction conditions to restore the alkene. Vicinal dibromides can also be converted to alkynes.
The same mechanism described above is followed
in this reaction. However, the first stage of the mechanism should involve the
nucleophilic alkene reacting with an electrophilic centre, and yet there is no
obvious electrophilic center in bromine. The bond between the two bromine atoms
is a covalent σ bond with both electrons equally shared
between the bromine atoms.
If there is no electrophilic center, how can a
molecule like bromine react with a nucleophilic alkene? The answer lies in the
fact that the bromine molecule approaches end-on to the alkene double bond and
an electrophilic center is induced (Fig.
7). Since the alkene double bond is electron rich it repels the elec-trons
in the bromine molecule and this results in a polarization of the Br–Br bond
such that the nearer bromine becomes electron deficient (electrophilic). Now
that an electrophilic center has been generated, the mechanism is the same as
before.
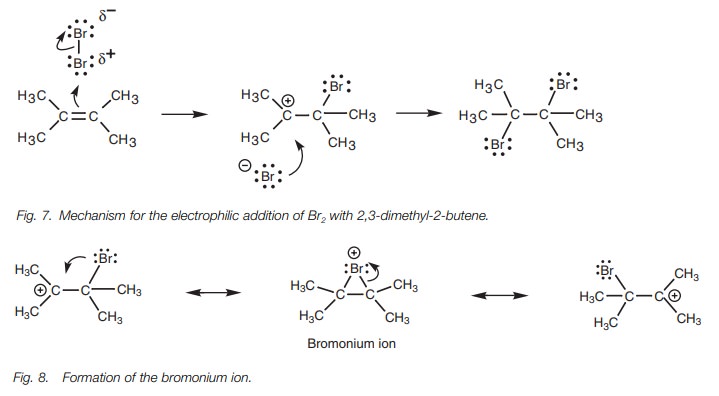
There is more to this mechanism than meets the
eye. The carbocation interme-diate can be stabilized by neighboring alkyl
groups through inductive and hyper-conjugation effects. However, it can also be
stabilized by sharing the positive charge with the bromine atom and a second
carbon atom (Fig. 8).
The positively charged carbon is an electrophilic center. The bromine is a weak nucleophilic center. A neutral halogen does not normally act as a nucleophile, but in this case the halogen is held close to the carbocation making reaction more likely.
Once the lone pair
of electrons on bromine is used to form a bond to the carbocation, a bromonium ion is formed where the
bromine gains a positive charge. The mechanism can go in reverse to regenerate
the origi-nal carbocation. Alternatively, the other carbon–bromine bond can
break with both electrons moving onto the bromine. This gives a second
carbocation where the other carbon bears the positive charge. Thus, the
positive charge is shared between three different atoms and is further
stabilized.
Evidence for the existence of the bromonium ion
is provided from the observation that bromine adds to cyclic alkenes (e.g.
cyclopentene) in an anti-stereochemistry
(Fig. 9). In other words, each
bromine adds to opposite faces of the alkene to pro-duce only the trans isomer. None of the cis isomer is formed. If the
intermediate was a carbocation, a mixture of cis and trans isomers
would be expected since the second bromine could add from either side. With a
bromonium ion, the second bromine must approach from the opposite side.
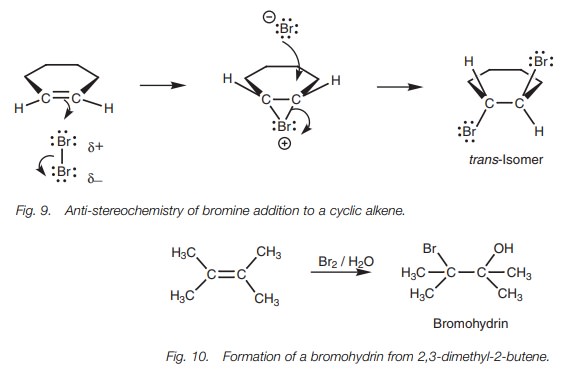
The reaction of an alkene with a halogen such
as bromine and chlorine normally gives a vicinal dihalide. However, if the
reaction is carried out in water as solvent, the product obtained is a
halohydrin where the halogen adds to one end of the double bond and a hydroxyl
group from water adds to the other (Fig.
10).
In this reaction, the first stage of the mechanism proceeds as normal, but then water acts as a nucleophile and ‘intercepts’ the carbocation intermediate (Fig. 11). Since water is the solvent, there are far more molecules of it present compared to the number of bromide ions generated from the first stage of the mechanism.
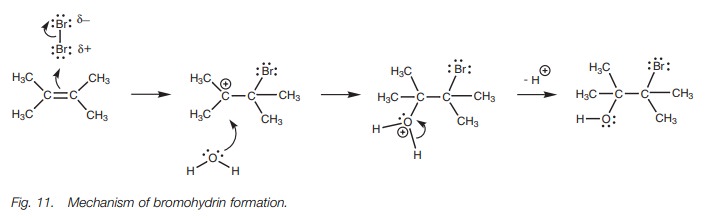
Water uses a lone pair of electrons on oxygen
to form a bond to the carbocation. As a result, the oxygen effectively ‘loses’
an electron and gains a positive charge. This charge is lost and the oxygen
regains its second lone pair when one of the O–H bonds breaks and both
electrons move onto the oxygen.
Alkenes to alcohols
Alkenes can be converted to alcohols by
treatment with aqueous acid (sulfuric orphosphoric acid; Fig. 12). This
electrophilic addition reaction involves the additionof water
across the double
bond. The hydrogen
adds to one
carbon while ahydroxyl group adds to the other carbon.
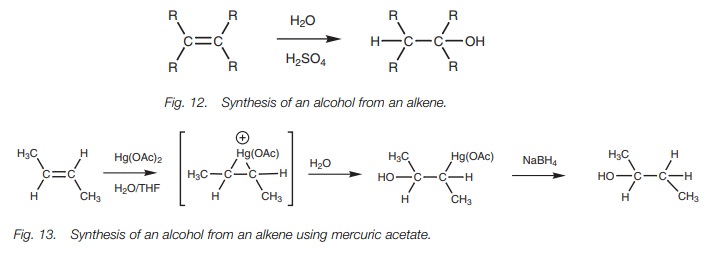
Sometimes the reaction conditions used in this
reaction are too harsh since heat- ing is involved and rearrangement reactions
can take place. A milder method which gives better results is to treat the
alkene with mercuric acetate [Hg(OAc)2] then sodium borohydride (Fig. 13). The
reaction involves electrophilic addition of the mercury reagent to form an
intermediate mercuronium ion. This reacts with water to give an organomercury
intermediate. Reduction with sodium borohy- dride replaces the mercury
substituent with hydrogen and gives the final product (Fig. 13)
Alkenes can also be converted to alcohols by hydroboration.
Alkenes to ethers
A similar reaction to the mercuric
acetate/sodium borohydride synthesis of alcohols allows the conversion of
alkenes to ethers. In this case, mercuric trifluoracetate is used (Fig. 14).
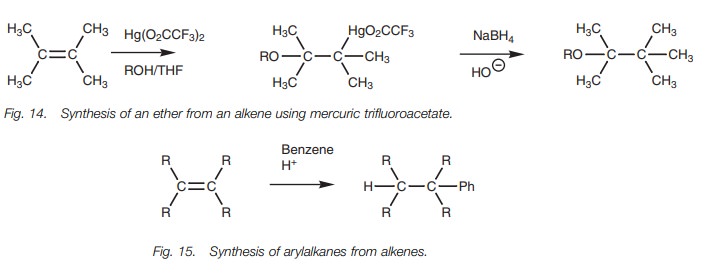
Alkenes to arylalkanes
The reaction of an aromatic ring such as benzene with an alkene under acid conditions results in the formation of an arylalkane (Fig. 15). As far as the alkene is concerned this is another example of electrophilic addition involving the addi-tion of a proton to one end of the double bond and the addition of the aromatic ring to the other.
As far as the
aromatic ring is concerned this is an example of an electrophilic substitution
reaction called the Friedel–Crafts
alkylation.
Related Topics