Chapter: Organic Chemistry: Alkenes and alkynes
Electrophilic addition to unsymmetrical alkenes
ELECTROPHILIC ADDITION TO UNSYMMETRICAL ALKENES
Key Notes
Addition of hydrogen halides
The
addition of a hydrogen halide to an unsymmetrical alkene can result in two different
products. These products are not formed in equal amounts. Markovnikov’s rule
states that ‘in the addition of HX to an alkene, the hydrogen atom adds to the
carbon atom that already has the greater num-ber of hydrogen atoms’. This
produces the more substituted alkyl halide.
Carbocation stabilities
The
favored product arising from addition of a hydrogen halide to an unsymmetrical
alkene will be formed from the more stable of the two pos-sible carbocations.
The more stable carbocation will have more alkyl groups attached to the
positive center.
Addition of halogens
Different
products are not possible from the reaction of a halogen with an unsymmetrical
alkene unless water is used as a solvent, in which case a hydroxyl group ends
up on the more substituted carbon. This demonstrates that the bromonium ion
does not share the positive charge equally amongst the bromine and the two
carbons.
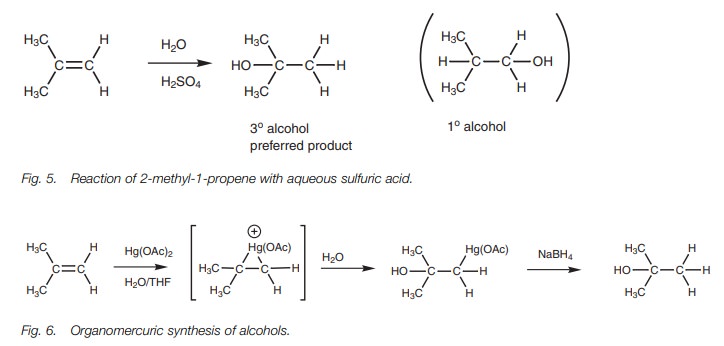
Addition of water
The more
substituted alcohol is the preferred product from the acidic hydrolysis of an
alkene as well as from the organomercuric synthesis of alcohols.
Addition of hydrogen halides
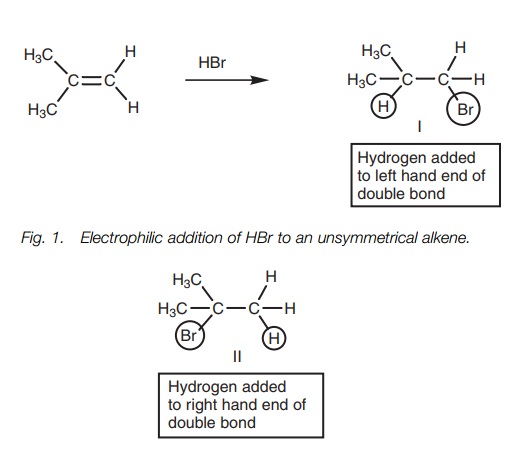
The reaction of a symmetrical alkene with
hydrogen bromide produces the same
product regardless of whether the hydrogen of HBr is added to one end of the double bond or the other. However, this is not the case with unsymmetrical alkenes (Fig. 1). In this case, two different products are possible. These are not formed to an equal extent and the more substituted alkyl halide (II) is preferred.
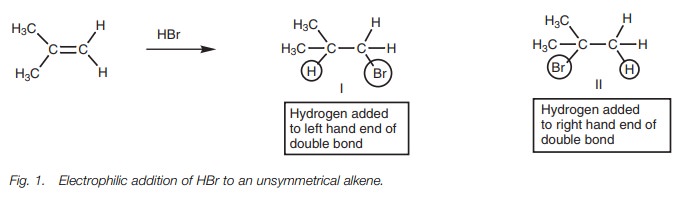
The reaction proceeds in a Markovnikov sense with hydrogen ending
up on the least substituted position and the halogen ending up on the most
substituted posi-tion. Markovnikov’s rule states that ‘in the addition of HX to
an alkene, the hydro-gen atom adds to the carbon atom that already has the
greater number of hydrogen atoms’. This produces the more substituted alkyl
halide.
Carbocation stabilities
This reaction can be rationalized by proposing
that the carbocation intermediate leading to product II is more stable than the
carbocation intermediate leading to product I (Fig. 2). It is possible to predict the more stable carbocation by
counting the number of alkyl groups attached to the positive center. The more
stable carbocation on the right has three alkyl substituents attached to the
positively charged carbon whereas the less stable carbocation on the left only
has one such alkyl substituent. The reasons for this difference in stability,
but the result is summarized by Markovnikov’s rule.
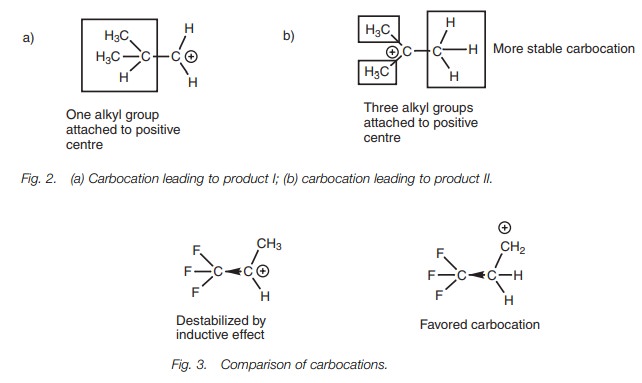
However, Markovnikov’s rule does not always hold true. For example, the reac-tion of CF3CH=CH2 with HBr gives CF3CH2CH2Br rather than CF3CHBrCH3. Here, the presence of electron-withdrawing fluorine substituents has a destabiliz-ing influence on the two possible intermediate carbocations (Fig. 3). The destabil-izing effect will be greater for the more substituted carbocation since the carbocation is closer to the fluorine substituents and so the favoured carbocation is the least substituted one in this case.
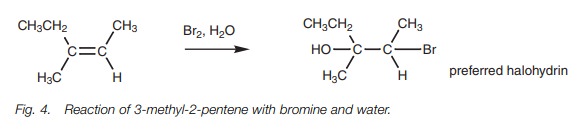
Addition of halogens
There is no possibility of different products when a halogen such as bromine or chlorine is added to an unsymmetrical alkene. However, this is not the case if water is used as a solvent. In such cases, the halogen is attached to the least sub-stituted carbon and the hydroxyl group is attached to the more substituted carbon (Fig. 4). This result can be explained by proposing that the bromonium ion is not symmetrical and that although the positive charge is shared between the bromine and the two carbon atoms, the positive charge is greater on the more substituted carbon compared with the less substituted carbon.
Addition of water
With unsymmetrical alkenes, the more
substituted alcohol is the preferred product (Fig. 5).
The same holds true for the organomercuric
synthesis of alcohols (Fig. 6).
Related Topics