Chapter: Organic Chemistry: Alkenes and alkynes
Reduction and oxidation of alkenes
REDUCTION AND OXIDATION OF ALKENES
Key Notes
Alkenes to alkanes
Alkenes
can be converted to alkanes by reduction with hydrogen gas. A metal catalyst is
necessary in order to lower the free energy of activation.
Alkenes to aldehydes and ketones
Treating
an alkene with ozone then with zinc and water results in cleavage of the alkene
across the double bond to give two carbonyl compounds (ketones and/or
aldehydes). The reaction is known as an ozonolysis and is an example of an
oxidation reaction.
Alkenes to carboxylic acids and ketones
Alkenes
can be cleaved by oxidation with hot alkaline potassium perman-ganate. The
products obtained are carboxylic acids and/or ketones depend-ing on the
substituents present on the alkene.
Alkenes to 1,2-diols
Alkenes
can be converted to 1,2-diols (or glycols) by reaction with osmium tetroxide or
by reaction with cold alkaline potassium permanganate. In both cases, the two
hydroxyl groups are added to the same face of the alkene – syn-hydroxylation. Osmium tetroxide gives better yields, but is
more toxic.
Alkenes to epoxides
Treatment
of alkenes with a peroxyacid (RCO3H) results in the formation of an
epoxide. The reaction is a one-step process without intermediates. Epoxides can
also be obtained in a two-step process via a halohydrin.
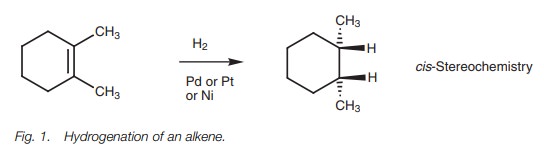
Alkenes to alkanes
Alkenes
are converted to
alkanes by treatment
with hydrogen over a finely divided metal catalyst such as
palladium, nickel, or platinum (Fig. 1).
This is an addition reaction since
it involves the addition of hydrogen atoms to each end of the double bond. It
is also called a catalytic hydrogenation
or a reduction reaction.
The catalyst is crucial since the reaction will
not take place at room temperature in its absence. This is because
hydrogenation has a high free energy of
activation (∆G1*) (Fig. 2). The role of the catalyst is to
bind the alkene and the hydrogen to a
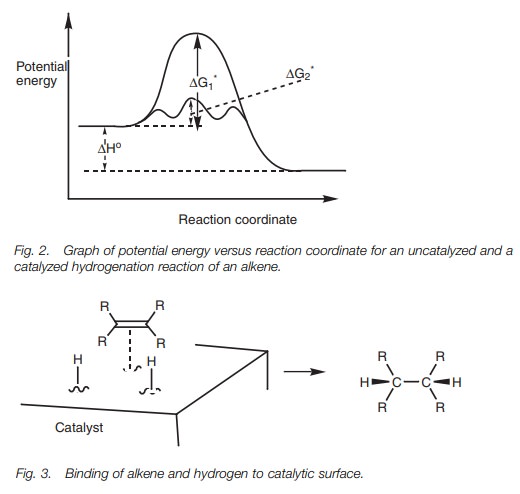
This results in a much lower energy of activation (∆G2*) allowing the reaction to proceed under much
milder conditions. The catalyst itself is unchanged after the reaction and can
be used in small quantity.
Both the hydrogen and the alkene are bound to
the catalyst surface before the hydrogen atoms are transferred, which means
that both hydrogens are added to the same side of the double bond (see Fig. 3) – syn-addition. Note that
the hydro-gen molecule is split once it has been added to the catalyst.
Alkenes to aldehydes and ketones
Treating an alkene with ozone (Fig. 4) results in oxidation of the alkene and formation of an initial ozonide which then rearranges to an
isomeric ozonide. This second ozonide is unstable and potentially explosive and
so it is not usually iso-lated. Instead, it is reduced with zinc and water
resulting in the formation of two separate molecules.
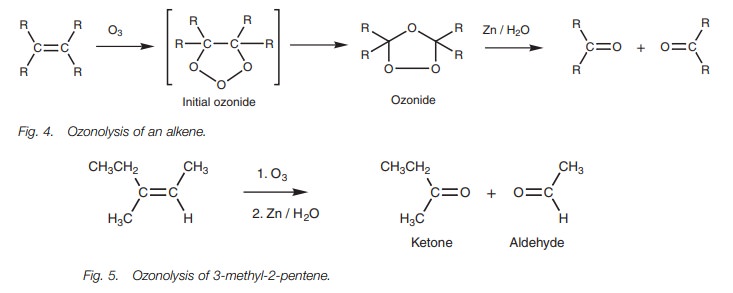
The alkene is split across the double bond to give two carbonyl compounds. These will be ketones or aldehydes depending on the substituents present. For example, 3-methyl-2-pentene gives an aldehyde and a ketone (Fig. 5).
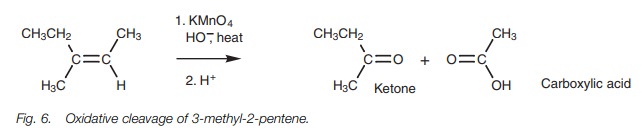
Alkenes to carboxylic acids and ketones
Alkenes can be oxidatively cleaved with hot
permanganate solution to give carboxylic acids and/or ketones (Fig. 6). The products obtained depend on
the substituents present on the alkene.
Alkenes to 1,2-diols
The reaction of alkenes with osmium tetroxide
(OsO4) is another example of an oxidation reaction (Fig. 7). However, in this case the
alkene is not split. Instead, a 1,2-diol is obtained – also known as a glycol.
The reaction involves the formation of a cyclic intermediate where the osmium
reagent is attached to one face of the alkene. On treatment with sodium
bisulfite, the intermediate is cleaved such that the two oxygen atoms linking
the osmium remain attached. This results in both alcohols being added to the
same side of the double bond – syn-hydroxylation.
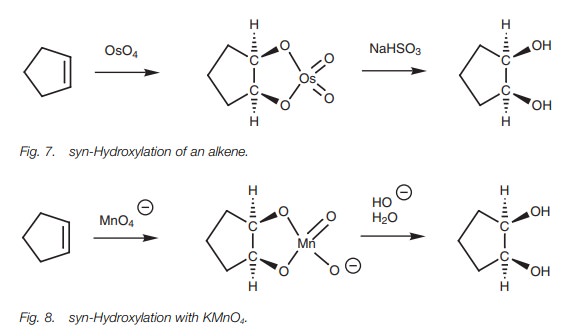
The same reaction can also be carried out using
cold alkaline potassium per- manganate (KMnO4) followed by treatment
with aqueous base (Fig. 8). It is
impor- tant to keep the reaction cold since potassium permanganate can cleave
the diol by further oxidation (Fig. 6).
The reaction works better with osmium tetroxide. However, this is a highly toxic and expensive reagent and has to be handled with care. Anti-hydroxylation of the double bond can be achieved by forming an epoxide, then carrying out an acid-catalyzed hydrolysis.
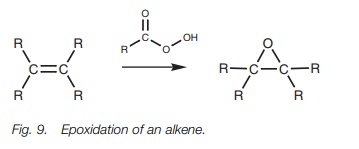
Alkenes to epoxides
Treatment of an alkene with a peroxyacid (RCO3H) results
in the formation of an epoxide (Fig. 9).
m-Chloroperoxybenzoic acid is one of
the most frequently used peroxyacids for this reaction. The reaction is unusual
in that there is no carboca-tion intermediate, and involves a one-step process
without intermediates.
Related Topics