Chapter: Organic Chemistry: Alkenes and alkynes
Hydroboration of alkenes
HYDROBORATION OF ALKENES
Key Notes
Reaction
Alkenes
can be converted to alcohols by treatment with diborane followed by hydrogen
peroxide. With unsymmetrical alkenes, the least substituted alcohol is obtained
in contrast to the electrophilic addition of water where the most substituted
alcohol is obtained.
Mechanism
The
mechanism involves an initial π complex between the alkene and BH3. A
four-centered transition state is then formed where the π bond of the alkene and one of the B–H bonds
is partially broken and the bonds linking H and B to the alkene are partially
formed. There is an imbalance of charge in the transition state, resulting in
one of the carbon atoms being slightly positive. The reaction proceeds such
that the most substituted carbon bears the partial charge and this results in
the boron adding to the least substi- tuted carbon. Oxidation with hydrogen
peroxide involves a hydroperoxide molecule bonding to boron, followed by
migration of an alkyl group from boron to oxygen. This is repeated twice more
to form a trialkyl borate which is hydrolyzed to give the final alcohol.
Reaction
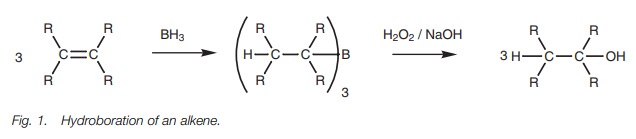
Alcohols can be generated from alkenes by
reaction with diborane (B2H6 or BH3),
followed by treatment
with hydrogen peroxide
(Fig. 1). The first
part of the reaction involves the splitting of a B–H
bond in BH3 with the hydrogen joining one end of the alkene and the boron
joining the other. Each of the B–H bonds is split in this way such that each
BH3 molecule reacts with three alkenes
to give an organoborane intermediate where boron is linked to three alkyl
groups. This can then be oxidized with alkaline hydrogen peroxide to produce
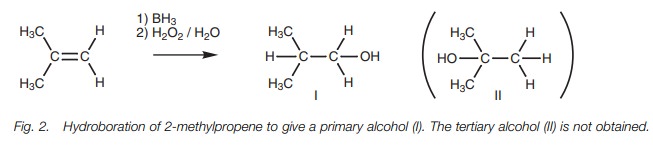
the alcohol. With unsymmetrical alkenes, the least substituted alcohol is
obtained (anti- Markovnikov; Fig. 2) and so the organoborane reaction is
complementary to the electrophilic addition reaction with aqueous acid. Steric
factors appear to play a role in controlling this preference with the boron
atom preferring to approach the least sterically hindered site. Electronic
factors also play a role as described in the mechanism below.
Mechanism
The mechanism (Fig. 3) involves the alkene π bond interacting with the empty p orbital of boron to form a π complex. One of BH3’s hydrogen atoms is then
transferred to one end of the alkene as boron itself forms aσ bond to the other end. This takes place through a four-centered
transition state where the alkene’s π bond and the B–H bond are partially broken,
and the eventual C–H and C–B bonds are partially formed. There is an imbalance
of electrons in the transition state which results in the boron being slightly
negative and one of the alkene carbons being slightly positive. The carbon best
able to handle this will be the most substituted carbon and so the boron will
end up on the least substituted carbon. (Note that boron has six valence
electrons and is electrophilic. Therefore, the addition of boron to the least
substituted position actually follows Markovnikov’s rule.) Since subsequent
oxidation with hydrogen peroxide replaces the boron with a hydroxyl group, the
eventual alcohol will be on the least substituted carbon. Fur-thermore, the
addition of the boron and hydrogen atoms take place such that they are on the
same side of the alkene. This is called syn-addition.
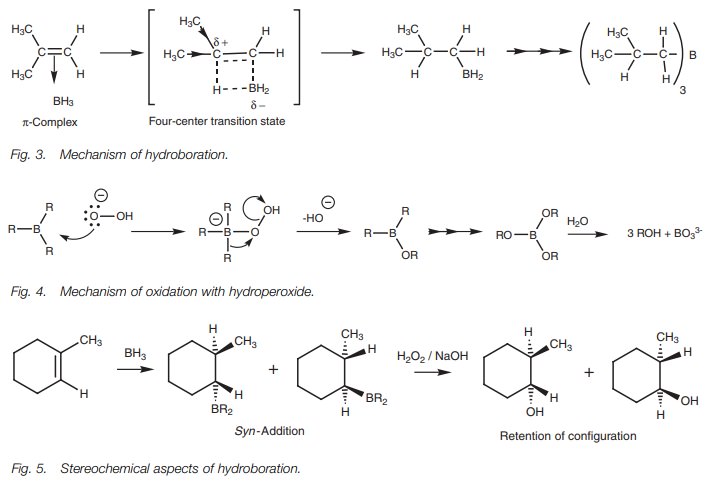
The mechanism of oxidation (Fig. 4) involves addition of the hydroperoxide to the electron deficient boron to form an unstable intermediate which
then rearranges such that an alkyl group migrates from the boron atom to the
neighboring oxygen and expels a hydroxide ion. This process is then repeated
for the remaining two hydrogens on boron and the final trialkyl borate B(OR)3
can then be hydrolyzed with water to give three molecules of alcohol plus a
borate ion.
The mechanism of oxidation takes place with
retention of stereochemistry at the alcohol’s carbon atom and so the overall
reaction is stereospecific (Fig. 5).
Note that the reaction is stereospecific such that the alcohol group is trans to the methyl group in the
product. However, it is not enantiospecific and both enantiomers are obtained
in equal amounts (a racemate).
Related Topics