Chapter: 12th Chemistry : UNIT 12 : Carbonyl Compounds and Carboxylic Acids
Physical properties of aldehydes and ketones
Physical properties of aldehydes and ketones
1. Physical State:
Formaldehyde is a gas at room temperature and acetaldehyde
is a volatile liquid. All other aldehydes and ketones upto to C11
are colourless liquids while the higher ones are solids.
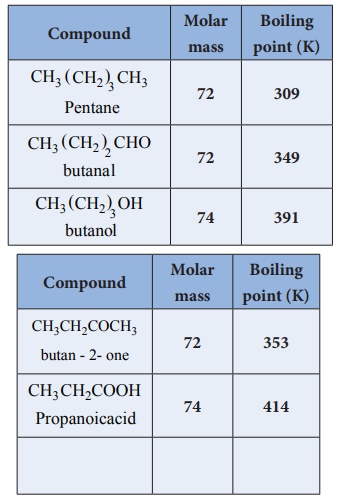
2. Boiling points
Aldehydes and ketones have relatively high boiling point as compared to
hydrocarbons and ethers of comparable molecular mass. It is due to the weak
molecular association in aldehydes and ketones arising out of the dipole-dipole
interactions.
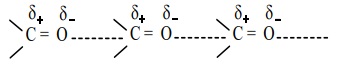
These dipole-dipole interactions are weaker than intermolecular
H-bonding. The boiling points of aldehydes and ketones are much lower than
those of corresponding alcohols and carboxylic acids which possess inter
molecular hydrogen bonding.
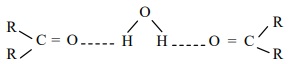
3. Solubility
Lower members of aldehydes and ketones like formaldehyde, acetaldehyde
and acetone are miscible with water in all proportions because they form
hydrogen bond with water.
Solubility of aldehydes and ketones decreases rapidly on increasing the
length of alkyl chain.
4. Dipolemoment:
The carbonyl group of aldehydes and ketones contains a double bond
between carbon and oxygen. Oxygen is more electronegative than carbon and it
attracts the shared pair of electron which makes the carbonyl group as polar
and hence aldehydes and ketones have high dipole moments.
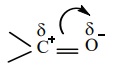
Related Topics