Preparation, Physical and Chemical properties - Esters | 12th Chemistry : UNIT 12 : Carbonyl Compounds and Carboxylic Acids
Chapter: 12th Chemistry : UNIT 12 : Carbonyl Compounds and Carboxylic Acids
Esters
Esters
Methods of preparation
1. Esterification
We have already learnt that treatment of alcohols with carboxylic acids
in presence of mineral acid gives esters. The reaction is carried to completion
by using an excess of reactant or by removing the water from the reaction
mixture.
2. Alcoholysis of Acid chloride or Acid anhydrides
Treatment of acid chloride or acid anhydride with alcohol also gives
esters
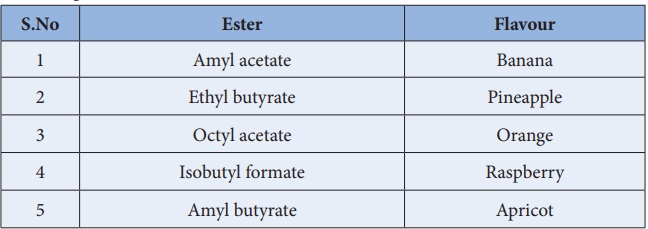
Physical Properties
Esters are colour less liquids or solids with characteristic fruity
smell. Flavours of some of the esters are given below.
Ester : Flavour
1. Amyl acetate: Banana
2. Ethyl butyrate: Pineapple
3. Octyl acetate: Orange
4. Isobutyl formate: Raspberry
5. Amyl butyrate: Apricot
Chemical Properties
1. Hydrolysis
We have already learnt that hydrolysis of esters gives alcohol and
carboxylic acid.
2. Reaction with alcohol ( Transesterification)
Esters of an alcohol can react with another alcohol in the presence of a
mineral acid to give the ester of second alcohol. The interchange of alcohol
portions of the esters is termed transesterification
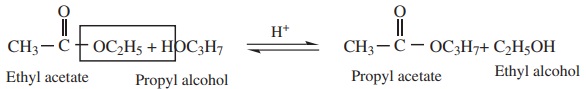
The reaction is generally used for the preparation of the esters of a
higher alcohol from that of a lower alcohol.
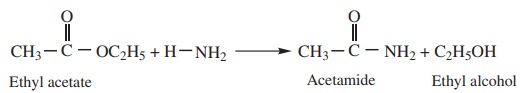
3. Reaction with ammonia (Ammonolysis)
Esters react slowly with ammonia to form amides and alcohol.
4. Claisen Condensation
Esters containing at least one ∝-
hydrogen atom undergo self condensation in the presence of a strong base such
as sodium ethoxide to form b- keto
ester.
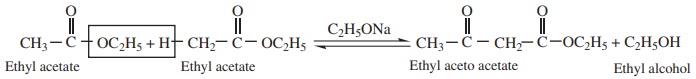
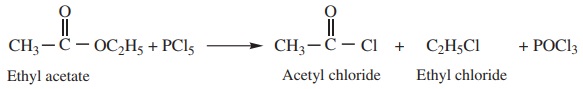
5. Reaction with PCl5
Esters react with PCl5 to give a mixture of acyl and alkyl
chloride
Evaluate yourself
Why is acid anhydride preferred to acyl chloride for
carrying out acylation reactions ?
Related Topics