Chapter: 12th Chemistry : UNIT 12 : Carbonyl Compounds and Carboxylic Acids
Methods of Preparation of carboxylic acids
Methods of Preparation of carboxylic acids
Some important methods for the preparation of carboxylic acids are as
follows :
1. From Primary alcohols and aldehydes
Primary alcohols and aldehydes can easily be oxidised to the
corresponding carboxylic acids with oxidising agents such as potassium
permanganate (in acidic or alkaline medium), potassium dichromate (in acidic
medium)
Example
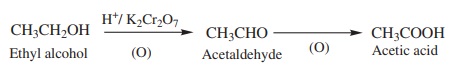
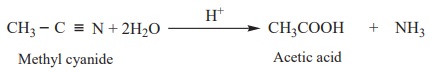
2. Hydrolysis of Nitriles
Nitriles yield carboxylic acids when subjected to hydrolysis with an
acid or alkali.
Example
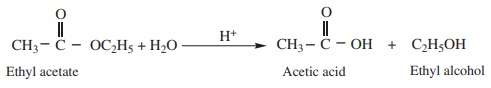
3. Acidic hydrolysis of esters
Esters on hydrolysis with dilute mineral acids yield corresponding
carboxylic acid
Example
4. From Grignard reagent
Grignard reagent reacts with carbon di oxide (dry ice) to form salts of
carboxylic acid which in turn give corresponding carboxylic acid after
acidification with mineral acid.
Example
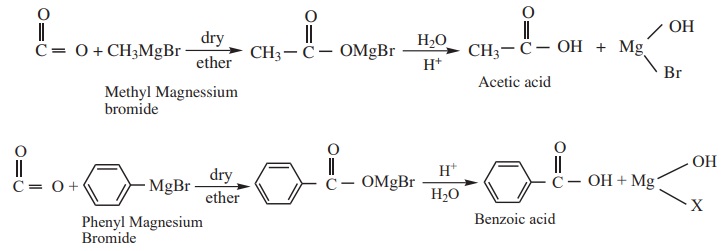
Formic acid cannot be prepared by Grignard reagent since the acid
contains only one carbon atom
5. Hydrolysis of acylhalides and anhydrides
a) Acid chlorides when hydrolysed with water give Carboxylic acids.
Example
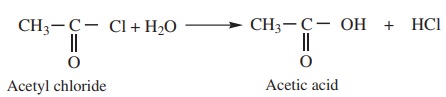
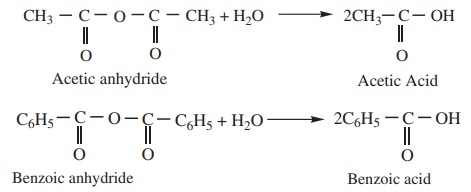
b) Acid anhydride when hydrolysed with water give corresponding
carboxylic acids.
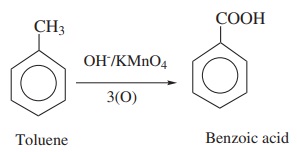
6. Oxidation of alkyl benzenes
Aromatic carboxylic acids can be prepared by vigorous oxidation of alkyl
benzene with chromic acid or acidic or alkaline potassium permanganate. The
entire side chain is oxidised to –COOH group irrespective of the length of the
side chain.
Example
Evaluate yourself
1) What happens when n-propyl benzene is oxidised using H+
/ KMnO4?
2) How will you prepare benzoic acid using Grignard
reagent.
Related Topics