Chapter: 12th Chemistry : UNIT 12 : Carbonyl Compounds and Carboxylic Acids
Physical Properties of carboxylic acids
Physical Properties of carboxylic acids.
i) Aliphatic carboxylic acid upto nine carbon atoms
are colour less liquids with pungent odour. The higher members are odourless
wax like solids.
ii) Carboxylic acids have higher boiling point than aldehydes, ketones
and even alcohols of comparable molecular masses. This is due to more
association of carboxylic acid molecules through intermolecular hydrogen
bonding.
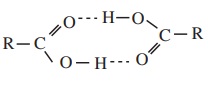
Inter
molecular hydrogen bonding
In fact, most of the carboxylic acids exist as dimer in its vapour
phase.
iii) Lower aliphatic carboxylic acids (up to four carbon) are miscible
with water due to the formation of hydrogen bonds with water. Higher carboxylic
acid are insoluble in water due to increased hydrophobic interaction of
hydrocarbon part. The simplest aromatic carboxylic acid, benzoic acid is
insoluble in water.
iv) Vinegar is 6 to 8% solution of acetic acid in water. Pure acetic
acid is called glacial acetic acid. Because it forms ice like crystal
when cooled. When aqueous acetic acid is cooled at 289.5 K, acetic acid solidifies and forms ice like crystals,
where as water remains in liquid state and removed by filtration. This process
is repeated to obtain glacial acetic acid.
Related Topics