Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Physical Properties of Haloalkanes
Physical Properties
1. Pure haloalkanes are colourless. Bromo and iodo alkanes are coloured in the presence of light.
2. Haloalkanes having one, two or three carbon atoms are in the gaseous state at normal temperature. Haloalkanes having more than three carbon atoms are liquids or solids.
3). Boiling point and Melting point
i) Haloalkanes have higher boiling point and melting point than the parent alkanes having the same number of carbons because the intermolecular forces of attraction (dipole – dipole interaction and vander Waals forces) are stronger in haloalkane.
ii) The boiling point and melting point of haloalkanes decreases with respect to the helogen in the following order.
Example
CH3I > CH3Br > CH3Cl > CH3F
iii) The boiling points of chloro, bromo and iodo alkanes increase with the increase in the number of halogen atoms.
For Example:
CCl4 > CHCl3 > CH2Cl2 > CH3Cl
iv) The boiling point and melting point of mono haloalkane increase with the increase in the number of carbon atoms.
Example
CH3CH2CH2Cl > CH3CH2Cl > CH3Cl
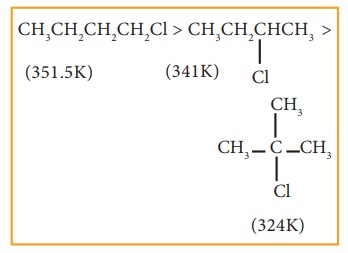
v) Among isomeric alkyl halides the boiling point decreases with the increase in branching in the alkyl group; with increase in branching, the molecule attains spherical shape with less surface area. As a result the inter molecular forces become weak, resulting in lower boiling points.
Example
4. Solubility
Haloalkanes are polar covalent compounds soluble in organic solvents, but insoluble in water because they cannot form hydrogen bonds with water molecules
5. Density
The density of liquid alkyl halides are higher than these of hydrocarbons of comparable molecular weight.
Related Topics