Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Nature of C- X bond in haloarenes
Nature of C- X bond in haloarenes
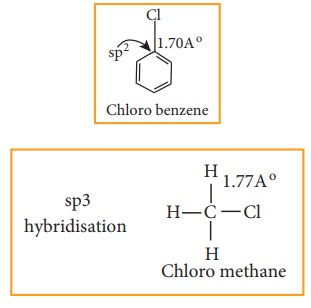
In halo arenes the carbon atom is sp2 hybridised. The sp2 hybridised orbitals are shorter and holds the electron pair of bond more tightly.
Halogen atom contains P-orbital with lone pair of electrons which interacts with π-orbitals of benzene ring to form extended conjugated system of π- orbitals. The delocalisation of these electrons give double bond character to C – X bond. The resonance structure of halobenzene is given as
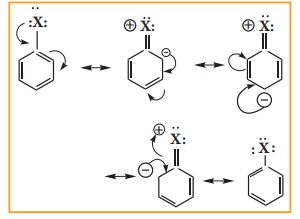
Due to this double bond character of C- X bond in haloarenes ,the C-X bond is shorter in length and stronger than in halo alkanes.
Example
Related Topics