Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Brief questions and answers: Chemistry: Haloalkanes and Haloarenes
Haloalkanes and Haloarenes | Chemistry
Answer the following questions
26. Classify the following compounds in the form of alkyl, allylic, vinyl, benzylic halides
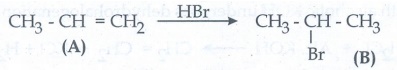
i. CH3 ŌĆō CH = CH ŌĆō Cl
ii. C6H5CH2I
iii. 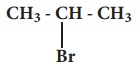
iv. CH2 = CH ŌĆō Cl
Ans:
a)
CH3-CH = CH-Cl - Vinylic
halide
b)
C6H5CH2I - Benzylic halide
c)  - Alkyl halide
- Alkyl halide
d)
CH2 = CH-Cl - Vinylic halide
27. Why chlorination of methane is not possible in dark?
Chlorination
of methane is a free radical substitution reaction. If chlorination of methane
is carriedout in dark, the energy will not be sufficient to undergo homolytic
fission of chlorine to produce chlorine free radical.
28. How will you prepare n propyl iodide from n-propyl bromide?
When
n-propyl bromide on heating with concentrate solution of sodium iodide in dry
acetone forms n-propyl iodide. This reaction is called Finkelstein reaction.
CH3CH2CH2Br
+ NaI __acetone_╬ö_ŌåÆ CH3CH2CH2I
+ NaBr
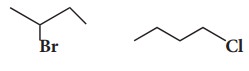
29. Which alkyl halide from the following pair is i) chiral ii) undergoes faster SN2 reaction?

30. How does chlorobenzene react with sodium in the presence of ether? What is the name of the reaction?
Chlorobenzene
react with sodium in the presence of dry ether gives biphenyl. This reaction is
called fittig reaction.
C6H5Cl
+ 2Na + Cl - C6H5 Ether╬ö__ŌåÆ
C6H5 - C6H5 +2NaCl
31. Give reasons for polarity of C-X bond in halo alkane.
In
haloalkane, halogen atom is more electronegative than carbon. So, the shared
pair of electron lies closer to the halogen atom. As a result, the halogen
carries a small negatives charge ╬┤ŌłÆ while the carbon carries a small
positive charge (╬┤+). Thus, C - X bond is a polar bond.
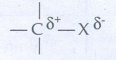
32. Why is it necessary to avoid even traces of moisture during the use of Grignard reagent?
Grignard
reagent is highly reactive towards moisture to give alkane
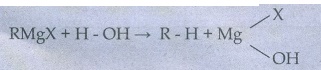
33. What happens when acetyl chloride is treated with excess of CH3MgI?
Acetyl
Chloride reacts with excess methyl magnesium iodide to give tert - butyl
alcohol.
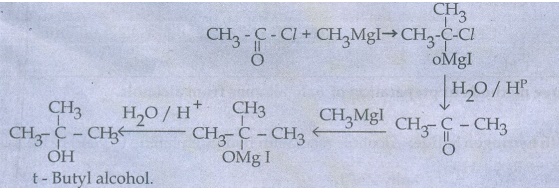
34. Arrange the following alkyl halide in increasing order of bond enthalpy of RX
CH3Br, CH3F, CH3Cl, CH3I
The
increasing order of bond enthalpy of alkyl halides are. CH3I < CH3Br
< CH3Cl < CH3F
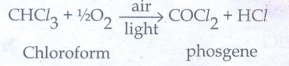
35. What happens when chloroform reacts with oxygen in the presence of sunlight?
Chloroform
undergoes oxidation in the presence of light and air to form a poisonous gas,
carbonyl chloride also known as phosgene.
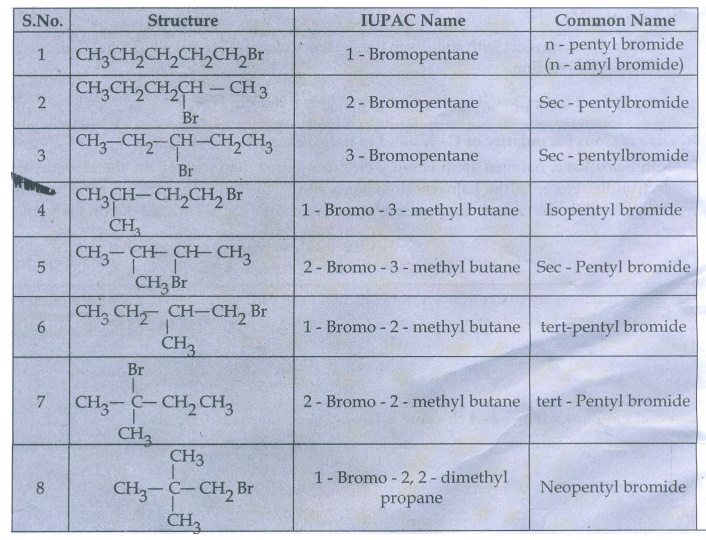
36. Write down the possible isomers of C5H11Br and give their IUPAC and common names.
The
possible isomers of C5H11Br are
37. Mention any three methods of preparation of haloalkanes from alcohols.
From
alcohols:
a)
Reaction with hydrogen halide: Alcohols react with hydrogen halide to
yield haloalkanes.
R
- OH + HX ŌåÆ R-X + H2O
Example
: CH3CH2OH + HCl ___anhy ZnCl2ŌåÆ
CH3CH2C1 + H2O
b)
Reaction with Phosphurous halides :
Alcohol
react with PX5 or PX3 to form haloalkanes.
R
- OH + PX5 ŌåÆ R - X + POX3+ HX
Example
: CH3CH2OH + PCl5 ŌåÆ CH3CH2Cl+
POCl3 + HCl
c)
Reaction with thionyl chloride :
Alcohols
are refuxed with SOCl2 in the presence of small amount of pyridine
to give chloroalkane. (Darzen's Reaction)
R
- OH + SOCl2 __pyridine__ŌåÆ R - Cl +
SO2 + HCl
Example
: CH3CH2OH + SOCl2 __pyridine__ŌåÆ
CH3CH3Cl + SO2 + HClŌĆā
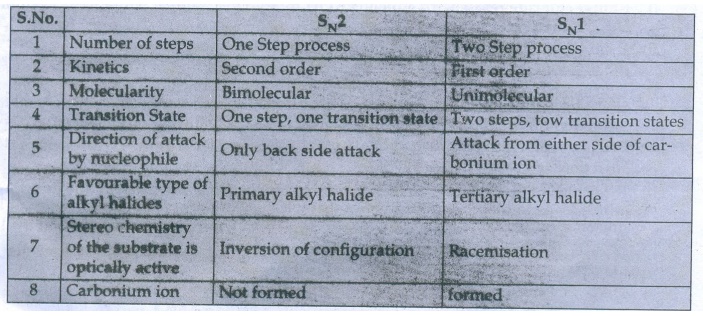
38. Compare SN1 and SN2 reaction mechanisms.
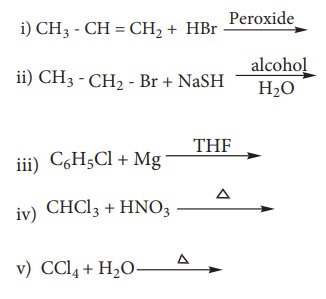
39. Reagents and the conditions used in the reactions are given below. Complete the table by writing down the product and the name of the reaction.
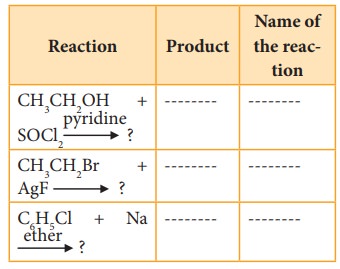
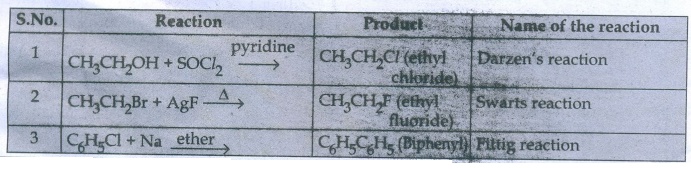
Answer:
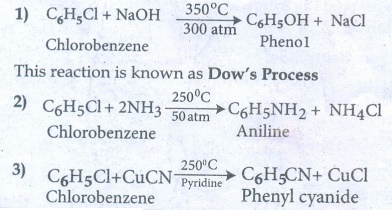
40. Discuss the aromatic nucleophilic substitutions reaction of chlorobenzene.
Aromatic
nucleophilic substitution reaction :
Halo
arenes do not undergo nucleophilic substitution reaction readily. This is due
to C-X bond in aryl halide is short and strong and also the aromatic ring is a
centre of high electron density.
The
halogen of haloarenes can be substituted by OHŌłÆ, NH3, or
CNŌłÆ with appropriate nucleophilic reagents at high temperature and
pressure.
Examples
:
41. Account for the following
i. t-butyl chloride reacts with aqueous KOH by SN1 mechanism while n-butyl chloride reacts with SN2 mechanism.
ii. p-dichloro benzene has higher melting point than those of o-and m-dichloro benzene.
i)
In general, SN1 reaction proceeds through the formation
of carbo cation, t-butyl chloride reacts with aqueous KOH, it form tert - butyl
chloride reacts with aqueous KOH, it form tert -butyl carbo cation which is
more stable. Hence, it follow SN1 mechanism.
In
the case of n-butylbromide, primary n-butyl carbo cation is formed which is
least stable so, it does not follow SN1 mechanism. Hence, it follow
SN2 mechanism.
ii)
P - dichloro benzene has high melting point than the other two isomers due its
symmetry which leads to more close packing of its molecules in the crystal
lattice. Hence, it has greater intermolecular force of attraction. Therefore,
it requires more energy for melting.
42. In an experiment ethyliodide in ether is allowed to stand over magnesium pieces. Magnesium dissolves and product is formed
a) Name the product and write the equation for the reaction.
b) Why all the reagents used in the reaction should be dry? Explain
c) How is acetone prepared from the product obtained in the experiment.
a)
Methyl magnesium is formed as product.
CH3I
+ Mg _ether_ŌåÆ CH3 MgI
b)
Generally, Grignard reagent are highly reactive towards moisture to form
alkane.
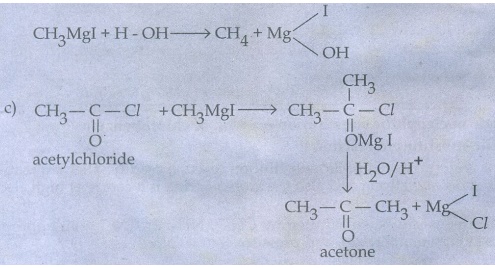
43. Write a chemical reaction useful to prepare the following:
i) Freon-12 from Carbon tetrachloride
ii) Carbon tetrachloride from carbon disulphide
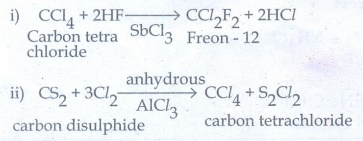
44. What are Freons? Discuss their uses and environmental effects
The
Chlorofluoro derivatives of methane and thane are called freons.
Uses
: Freons are used as
i)
refrigerants in refrigerators and air conditioners.
ii)
Propellant for aerosols and foams.
iii)
Propellant for foams to spray out deodorants, shaving creams and insecticides.
45. Predictthe products when bromoethane is treated with the following
![]()
![]() i. KNO2
i. KNO2
ii. AgNO2
i)
Bromoethane reacts with alcoholic KNO2 to form ethyl nitrite.
C2H5Br
+ KNO2 ŌåÆ C2H5 - O -N = O + KBr
ii)
Bromoethane react with alcoholic silver nitrite to form nitro ethane.
C2H5Br
+AgNO2 ŌåÆ C2H5 NO2+AgBr
46. Explain the mechanism of SN1 reaction by highlighting the stereochemistry behind it
SN1
Mechanism:
SN1
stands for unimolecular nucleophilic substitution
'S'
stands for substitution
ŌĆśNŌĆÖ
stands for nucleophilic
ŌĆś1ŌĆÖ
stands for unimolecular (one molecule is involved in the rate determining step)
The rate of the following SN1 reaction depends upon the
concentration of alkyl halide (RX) and is independent of the concentration of
the nucleophile (OHŌłÆ).
Ōł┤ Rate of
the reaciton = k [alkyl halide].
R
- Cl + OHŌłÆ ŌåÆ R - OH + ClŌłÆ
This
SN1 reaction follows first order kinetics ans occurs in two steps.
Let
us consider SN1 reaction mechanism by taking a reaction between
tertiary butyl bromide with aqueous KOH.
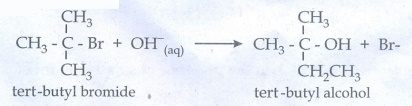
This
reaction takes place in two steps as shown below
Step
ŌłÆ1 Formation of carbocation
The
polar C - Br bond breaks forming a carboncation and bromide ion. This step is
slow and hence it is the rate determining step.
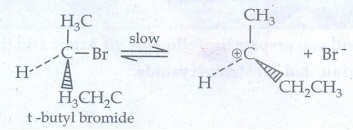
The
carbocation has 2 equivalent lobes of the vacant 2p orbital, so it can react
equally rapidly from either face.
Step
- 2 Nucleophilic attack on carbocation
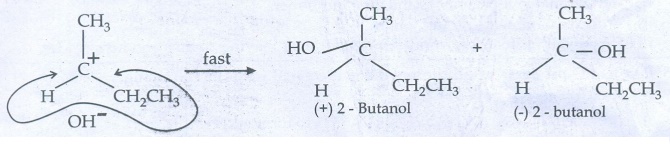
As
nucleophilic reagent OHŌłÆ can attack carbocation from both the sides,
to form equal proportion of dextro and levorotatory optically active isomers
which from optically inactive racemic mixture.
47. Write short notes on the the following
i. Raschig process
ii. Dows Process
iii. Darzens process
i)
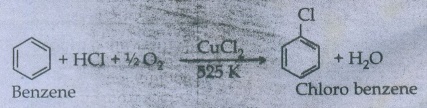
Raschig process
Chloro
benzene is commercially prepared by passing a mixture of benzene vapour, air
and HCl over heated cupric chloride.
This reaction is called Raschig process.
(ii)
Dows process
Reaction
of Chlorobenzene with caustic soda at 350┬░C and 300 atm gives phenol. This
reaction is used for large scale preparation as it known as Dows process.

(iii)
Darzens Process
Alcohols
are refluxed with SOCl2 in the presence of small amount of
pyridine to give chloro alkanes.
R
- OH + SOCl2 __Pyridine_ŌåÆ
R - Cl + SO2 + HCl
Example
CH3CH2OH
Ethanol + SOCl2 __Pyridine_ŌåÆ
CH3CH2Cl Chloroethane
+ SO2 + HCl
This
reaction is known as DarzenŌĆÖs reaction.
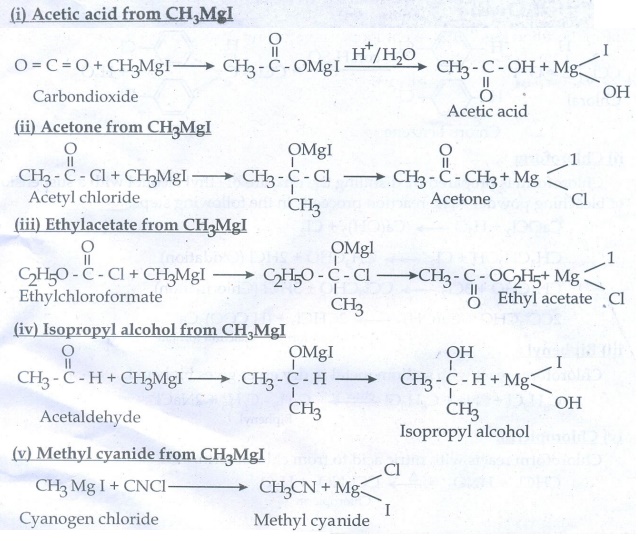
48. Starting from CH3MgI, How will you prepare the following?
i. Acetic acid
ii. Acetone
iii. Ethyl acetate
iv. Iso propyl alcohol
v. Methyl cyanide
49. Complete the following reactions
Answer:
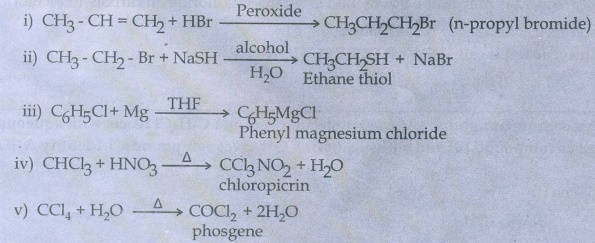
50. Explain the preparation of the following compounds
i) DDT
ii) Chloroform
iii) Biphenyl
iv) Chloropicrin
v) Freon-12
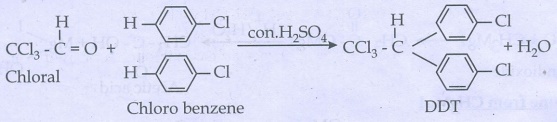
i)
DDT
DDT
can be prepared by heating a mixture of chlorobenzene with chloral (Trichlor)
acetaldehyde) in the presence of con.H2SO4.ŌĆā
ii) Chloroform
Chloroform is prepared by
distilling the mixture of ethyl alcohol with a suspension of bleaching powder -
The reaction proceeds in the following steps.
CaOCl2 + H2O
ŌåÆ Ca(OH)2 + Cl2
CH3CH2OH +
Cl2 ŌåÆ CH3CHO + 2HCl (Oxidation)
CH3CHO + 3Cl2
ŌåÆ CCl3CHO + 3HCI (Chlorinaiton)
2CC13CHO + Ca(OH)2
ŌåÆ 2CHCl3 Chloroform + (H COO)2Ca Calcium formate
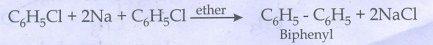
iii) Biphenyl:
Chloroform react with sodium
metal in dry ether gives biphenyl.
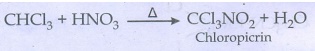
iv) Chloropicrin:
Chloroform reacts with nitric
acid to from chloropicrin.
v)
FreonŌłÆ12
FreonŌłÆ12
is prepared by the action of hydrogen fluoride on carbon tetrachloride in the
presence of antimony penta chloride (SbCl3)
This
reaction is called SWARTZ REACTION
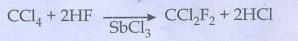
CCl4
+ 2HF __SbCl3ŌåÆ CCl2F2 + 2HCl
51. An organic compound (A) with molecular formula C2H5Cl reacts with KOH gives compounds (B) and with alcoholic KOH gives compound (C). Identify (A),(B), and (C)
Given:
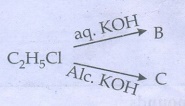
Solution:
As
per molecular formula, compound (A) is ethyl chloride. Ethyl chloride (A) reacts
with aqueous KOH gives ethyl alcohol (B).
CH3CH2C1
(A) +
aq. KOH ŌåÆ CH3CH2OH (B) +
KCl
Ethyl chloride (A) with alcoholic
KOH undergoes dehydrohalogenation to give ethylene (C).
CH3CH2Cl (A) + Alc. KOH ŌåÆ CH2 = CH2
(C) +
KCl + H2O
Result:
Compound
: Formula, Name
A
: CH3CH2Cl Ethyl chloride
B
: CH3CH2OH Ethyl alcohol
C
: CH2 = CH2 Ethylene
52. Simplest alkene (A) reacts with HCl to form compound (B).Compound (B) reacts with ammonia to form compound (C) of molecular formula C2H7N.Compound (C) undergoes carbylamine test. Identify (A), (B), and (C).
Given
:
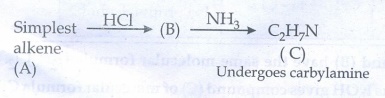
Solution
:
Simplest
alkene (A) means, it is ethylene. Ethylene (A) react with HCl to give form
ethyl chloride (B)
CH2
= CH2 (A) + HCl ŌåÆ CH3CH2Cl (B)
Ethylchloride
(B) reacts with ammonia to form ethylamine (C)
CH3CH2Cl
(B) +
NH3 ŌåÆ CH3CH2NH2 (C) +
HCl
Result
:
Compound : Formula & Name
A
: CH2 = CH2 Ethylene
B
: CH3CH2Cl Ethyl chloride
C
: CH3CH2NH2 Ethylamine
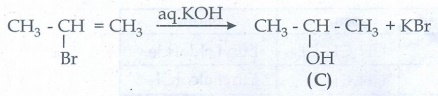
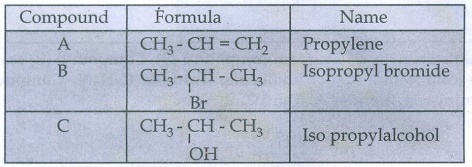
53. A hydrocarbon C3H6 (A) reacts with HBr to form compound (B). Compound (B) reacts with aqueous potassium hydroxide to give (C) of molecular formula C3H6O.what are (A) (B) and (C). Explain the reactions.
Solution:
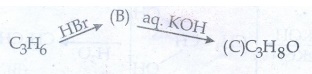
As per
molecular formula, A must be alkene ie., propene compound (A) reacts with HBr
forms isopropyl bromide (B)
Isopropyl
bromide (B) reacts with aq. KOH give isopropyl alcohol (C)
Result:
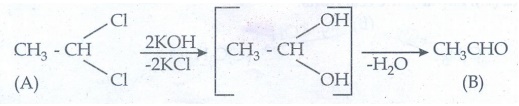
54. Two isomers (A) and (B) have the same molecular formula C2H4Cl2. Compound (A) reacts with aqueous KOH gives compound (C) of molecular formula C2H4O. Compound (B) reacts with aqueous KOH gives compound (D) of molecular formula C2H6O2. Identify (A),(B),(C) and (D).
Given:
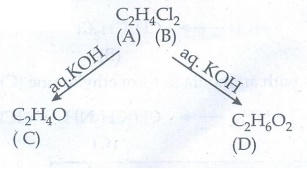
Solution:
Compound
(A) is dihalide. So, the possible dihalide will be vicinal dihalide and
gemdihalide. The possible two isomers are
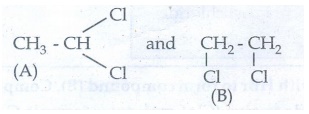
Compound
(A) 1, 1-Dichloro ethane on hydrolysis with aq.KOH give aldehyde (B)
Compound
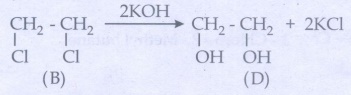
(B), 1, 2 - Dichloro ethane on hydrolysis with aq.KOH gives diols (D)ŌĆā
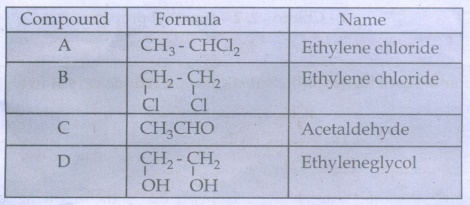
Result:
Related Topics