Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Organo metallic Compounds
Organo
metallic Compounds
Organo
metallic compounds are organic compounds in which there is a direct carbon
ŌĆōmetal bond
Example
CH3 Mg I - Methyl magnesium iodide
CH3
CH2 Mg Br - Ethyl magnesium bromide
The
carbon - magnesium bond in Grignard reagent is covalent but highly polar. The
carbon atom is more electro negative than magnesium. Hence, the carbon atom has
partial negative charge and the magnesium atom has partial positive charge
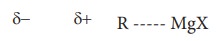
Preparation
When
a solution of alkyl halide in ether is allowed to stand over pieces of
magnesium metal, the metal gradually dissolves and alkyl magnesium halide
(Grignard reagent) is formed. All the reagents used should be pure and dry
Example
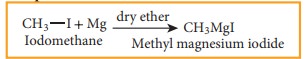
Uses of Grignard reagent
Grignard
reagents are synthetically very useful compounds. These reagents are converted
to various organic compounds like alcohols, carboxylic acids, aldehydes and
ketones. The alkyl group being electron rich acts as a carbanion or a
nucleophile. They would attack polarized molecules at a point of low electron
density. The following reactions illustrate the synthetic uses of Grignard
reagent.
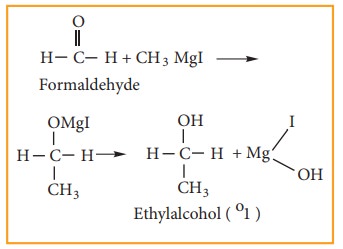
(1) Preparation of primary alcohol
Formaldehyde
reacts with Grignard reagent to give addition products which on hydrolysis
yields primary alcohol.
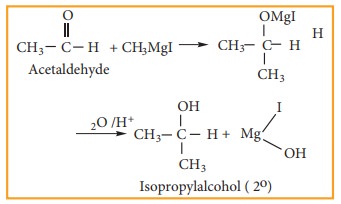
2) Preparation of secondary alcohol
Aldehydes
other than formaldehyde, react with Grignard reagent to give addition product
which on hydrolysis yields secondary alcohol.
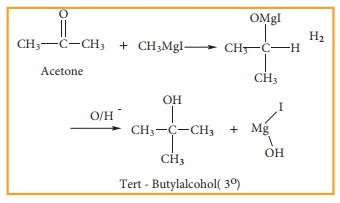
3) Preparation of Tertiary alcohol
Ketone
reacts with Grignard reagent to give an addition product which on hydrolysis
yields tertiary alcohols.
Example
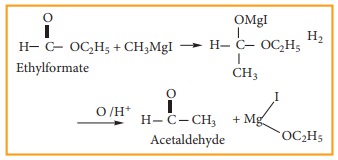
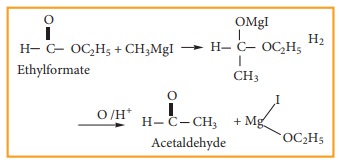
(4) Preparation of aldehyde
Ethyl
formate reacts with Grignard reagent to form aldehyde. However, with excess of
Grignard reagent it forms secondary alcohol.
Example
(5) Preparation of ketone
Acid
chloride reacts with Grignard reagent to form ketones. However, with excess of
Grignard reagent it forms tertiary alcohol.
Example
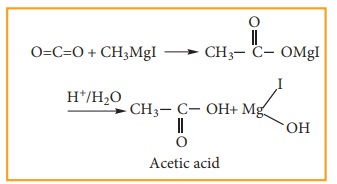
6) Preparation of carboxylic acids
Solid
carbon dioxide reacts with Grignard reagent to form addition product which on
hydrolysis yields carboxylic acids.
For Example
7) Preparation of esters
Ethylchloroformate
reacts with Grignard reagent to form esters.
Example
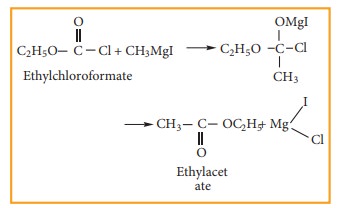
8) Preparation of higher ethers
Lower
halogenated ether reacts with Grignard reagent to form higher ethers.
Example
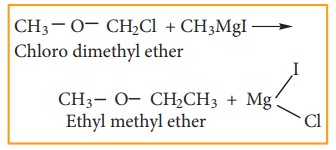
9) Preparation of alkyl cyanide
Grignard
reagent reacts with cyanogen chloride to from alkyl cyanide
Example
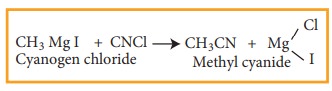
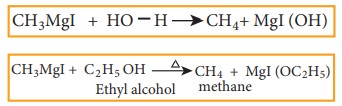
10) Preparation of Alkanes
Compounds
like water, alcohols and amines which contain active hydrogen atom react with
Grignard reagents to form alkanes.
Example
Related Topics