Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Chemical properties of Haloalkanes
Chemical properties
Haloalkanes are one of the most reactive classes of organic compounds. Their reactivity is due to the presence of polar carbon ŌĆō halogen bond in their molecules. The reactions of haloalkane may be divided into the following types
1. Nucleophilic substitution reactions
2. Elimination reactions
3. Reaction with metals
4. Reduction
1) Nucleophilic substitution reactions
We know that the C╬┤+ - X╬┤- present in halo alkane is polar and hence the nucleophilic reagents are attracted by partially positively charged carbon atoms resulting in substitution reactions.
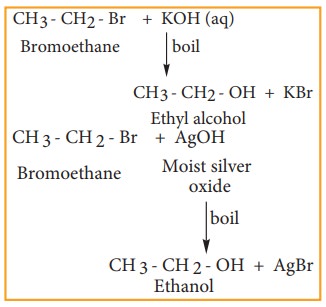
Reaction with aqueous alkali or moist silver oxide.(Hydrolysis)
Haloalkane reacts with aqueous solution of KOH or moist silver oxide (Ag2O/H2O) to form alcohols.
Example
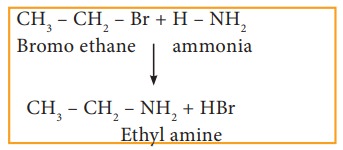
i) Reaction with alcoholic ammonia (Ammonolysis)
Haloalkanes react with alcoholic ammonia solution to form alkyl amines.
Example
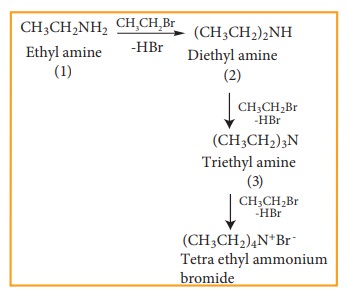
However, with excess of halo alkane, secondary and tertiary amines along with quartenary ammonium salts are obtained
Ambident Nucleophiles
Nucleophiles such as cyanide and nitrite ion which can attack nucleophilic centre from two sides are called ambident nucleophiles. These nucleophiles can attack with either of the two sides depending upon the reaction conditions and the reagent used.
ii) Reaction with alcoholic KCN
Haloalkanes react with alcoholic KCN solution to form alkyl cyanides.
Example
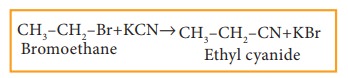
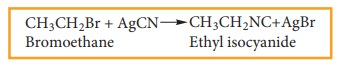
iii) Reaction with alcoholic AgCN
Haloalkanes react with alcoholic AgCN solution to form alkyl isocyanide.
Example
iv) Reaction with sodium or potassi-um nitrite
Haloalkanes react with alcoholic solution of NaNO2 or KNO2 to form alkyl nitrites.
Example
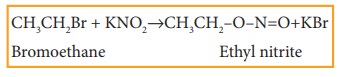
v) Reaction with silver nitrite
Haloalkanes react with alcoholic solution of AgNO2 to form nitro alkanes. Example
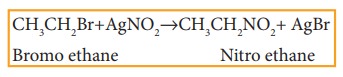
vi) Reaction with sodium or potassi-um hydrogen sulphide
Haloalkanes react with sodium or potassium hydrogen sulphide to form thio alcohols.
Example
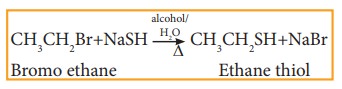
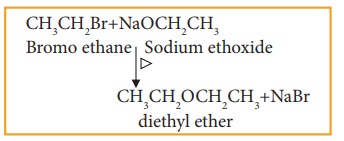
vii) Williamson ether synthesis
Haloalkane, when boiled with sodium alkoxide gives corresponding ethers.
Example
This method can be used to prepare mixed (unsymmetrical) ethers also.
Mechanism of Nucleophilic substitu-tion reaction
The mechanism of nucleophilic substitution reaction is classified as
a) Bimolecular Nucleophilic substitution reaction (SN2)
b) Unimolecular Nucleophilic substitution reaction (SN1)
SN2 Mechanism
The rate of SN2 reaction depends upon the concentration of both alkyl halide and the nucleophile.
Rate of reaction = k2 [alkylhalide] [nucleophile]
It follows second order kinetics and occurs in one step.
This reaction involves the formation of a transition state in which both the reactant molecules are partially bonded to each other. The attack of nucleophile occurs from the back side (i.e opposite to the side in which the halogen is attacked). The carbon at which substitution occurs has inverted configuration during the course of reaction just as an umbrella has tendency to invert in a wind storm. This inversion of configuration is called Walden inversion; after paul walden who first discovered the inversion of configuration of a compound in SN2 reaction.
SN2 reaction of an optically active haloalkane is always accompanied by inversion of configuration at the asymmetric centre. Let us consider the following example
When 2 - Bromooctane is heated with sodium hydroxide, 2 ŌĆō octanol is formed with invesion of configuration. (-) ŌĆō 2 ŌĆō Bromo octane is heated with sodium hydroxide (+) ŌĆō 2 ŌĆō Octanol is formed in which ŌĆō OH group occupies a position opposite to what bromine had occupied,
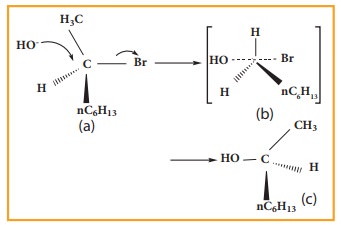
a) (-) 2 ŌĆō Bromo octane
b) Transition State
c) (+) 2 ŌĆō Octanol (product)
SN1 Mechanism
SN1 stands for unimolecular nucleophilic substitution
ŌĆśSŌĆÖ stands for substitution
ŌĆśNŌĆÖ stands for nucleophilic
ŌĆś1ŌĆÖ stands for unimolecular (one molecule is involved in the rate determining step)
The rate of the following SN1 reaction depends upon the concentration of alkyl halide (RX) and is independent of the concentration of the nucleophile (OHŌłÆ).
Hence Rate of the reaction = k[alkyl halide]
RŌłÆCl + OHŌłÆ ŌåÆ R ŌĆō OH + ClŌłÆ
This SN1 reaction follows first order kinetics and occurs in two steps.
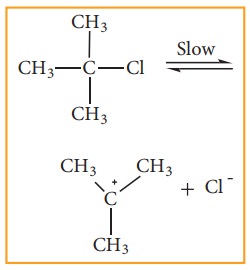
We understand SN1 reaction mechanism by taking a reaction between tertiary butyl bromide with aqueous KOH.
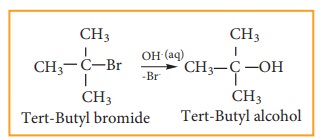
This reaction takes place in two steps as shown below
Step - 1 Formation of carbocation
The polar C - Br bond breaks forming a carbocation and bromide ion. This step is slow and hence it is the rate determining step.
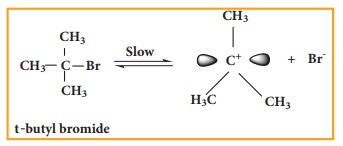
The carbocation has 2 equivalent lobes of the vacant 2p orbital, so it can react equally rapidly from either face
Step - 2
The nucleophile immediately reacts with the carbocation. This step is fast and hence does not affect the rate of the reactions.
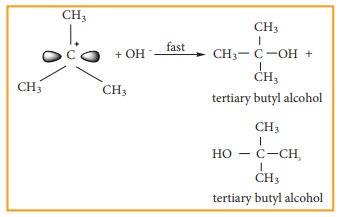
As shown above, the nucleophilic reagent OH- can attack carbocation from both the sides.
![]()
![]() In the above example the substrate tert-butyl bromide is not optically active, hence the obtained product is optically inactive. If halo alkane substrate is optically active then, the product obtained will be optically inactive racemic mixture. As nucleophilic reagent OH- can attack carbocation from both the sides, to form equal proportion of dextro and levorotatory optically active isomers which results in optically inactive racemic mixture.
In the above example the substrate tert-butyl bromide is not optically active, hence the obtained product is optically inactive. If halo alkane substrate is optically active then, the product obtained will be optically inactive racemic mixture. As nucleophilic reagent OH- can attack carbocation from both the sides, to form equal proportion of dextro and levorotatory optically active isomers which results in optically inactive racemic mixture.
Example
Hydrolysis of optically active 2 - bromo butane gives racemic mixture of ┬▒ butan-2-ol
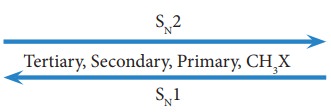
The order of reactivity of haloalkanes towards SN1 and SN2 reaction is given below. SN2 reaction
2) Elimination reactions
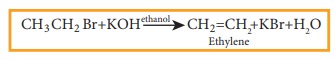
When a haloalkene containing a hydrogen on ╬▓ carbon is treated with an ethanolic solution of potassium hydroxide, an alkene is formed. In this reaction a double bond between ╬▒ and ╬▓ carbon is formed by releasing a halogen attached to a ╬▒ carbon and a hydrogen to a ╬▓ carbon of halo alkane. This reaction is called ╬▓ elimination reaction. (dehydrohalogenation).
Some haloalkanes yield a mixture of olefins in different amounts. It is explained by SaytzeffŌĆÖs Rule, which states that ŌĆśIn a dehydrohalogenation reaction, the preferred product is that alkene which has more number of alkyl groups attached to the doubly bonded carbon (more substituted double bond is formed)
Example
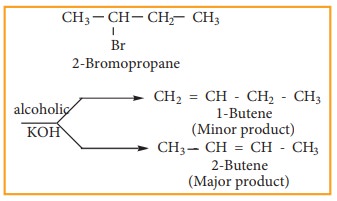
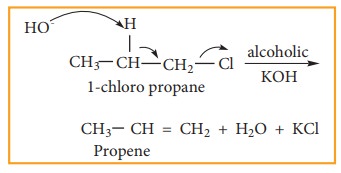
Elimination reactions may proceed through two different mechanisms namely E1 and E2
E2 reaction mechanism

The rate of E2 reaction depends on the concentration of alkyl halide and base
Rate = k [alkyl halide] [base]
It is therefore, a second order reaction. Generally primary alkyl halide undergoes this reaction in the presence of alcoholic KOH. It is a one step process in which the abstraction of the proton from the ╬▓ carbon and expulsion of halide from the ŌłØ carbon occur simultaneously. The mechanism is shown below.
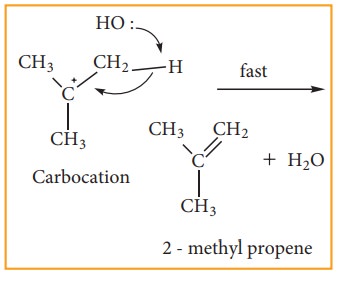
E1 reaction mechanism

Generally, tertiary alkyl halide which undergoes elimination reaction by this mechanism in the presence of alcoholic KOH. It follows first order kinetics. Let us consider the following elimination reaction.
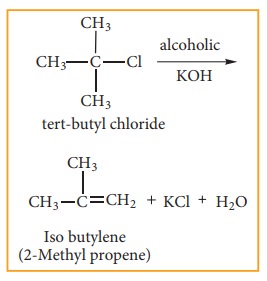
Step - 1 Heterolytic fission to yield a carbo-cation
Step - 2 Elimination of a proton from the - carbon to produce an alkene
3) Reaction with metals
Haloalkane reacts with metals, to form a compound containing carbon - metal bond known as organometallic compounds.
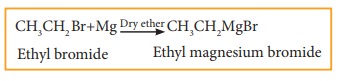
a) Grignard reaction
When a solution of halo alkane in ether is treated with magnesium, we get alkyl magnesium halide known as Grignard reagent.
Example
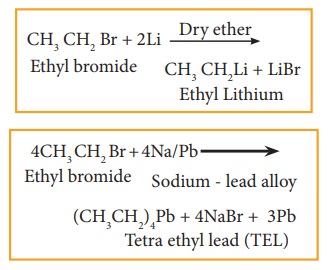
b) Reaction with active metals like so-dium, lead etc
![]()
![]() Haloalkane reacts with active metals like sodium, lead etc in the presence of dry ether to form organo metallic compounds.
Haloalkane reacts with active metals like sodium, lead etc in the presence of dry ether to form organo metallic compounds.
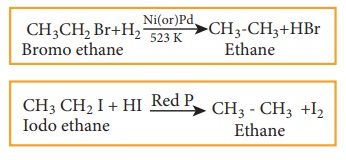
4) Reduction reactions
Haloalkanes are reduced to alkanes by treating with H2 in the presence of metal catalyst like nickel, palladium etc or with hydroiodic acid in the presence of red phosphorous.
The chemistry of haloalkane can be well understood by the following flowchart.
Related Topics