Nomenclature and isomerism, Preparation, Physical and Chemical properties, Mechanism, Uses, IUPAC name, structural formula | Chemistry - Haloarenes | 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Haloarenes
Haloarenes
Haloarenes
are the compounds X in which the halogen is directly attached to the
benzene ring.
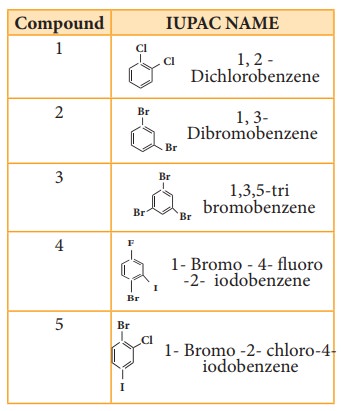
Nomenclature of haloarenes
In
the IUPAC nomenclature, the halo arenes are named by adding prefix halo before
the name of the aromatic hydrocarbon. For naming disubstituted arenes, the
relative position of the substituent 1,2; 1,3 and 1,4 are indicated by the
prefixes ortho, meta and para, respectively.
For
poly haloarenes the numbering should be done in such a way that the lowest possible
number should be given to the substituents and the name of the halogens are
arranged in alphabetic order.
Nomenclature
can be well understood from the following examples.
Nature of C- X bond in haloarenes
In
halo arenes the carbon atom is sp2 hybridised. The sp2
hybridised orbitals are shorter and holds the electron pair of bond more
tightly.
Halogen
atom contains P-orbital with lone pair of electrons which interacts with
π-orbitals of benzene ring to form extended conjugated system of π- orbitals.
The delocalisation of these electrons give double bond character to C – X bond.
The resonance structure of halobenzene is given as
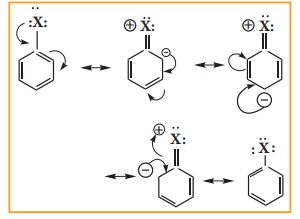
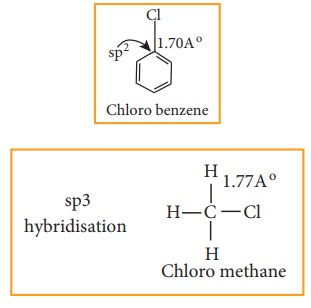
Due to this double bond character of C- X bond in haloarenes
,the C-X bond is shorter in length and stronger than in halo alkanes.
Example
Methods of preparation
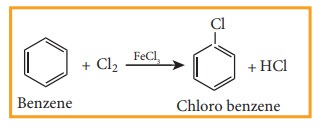
1) Direct halogenations
Chlorobenzene
is prepared by the direct chlorination of benzene in the presence of lewis acid
catalyst like FeCl3
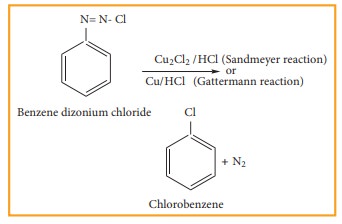
2) From benzene diazonium chloride
Chloro benzene is prepared by Sandmeyer reaction or Gattermann reaction using benzene
diazonium chloride.
(i) Sandmeyer reaction
When
aqueous solution of benzene diazonium chloride is warmed with Cu2Cl2
in HCl gives chloro benzene
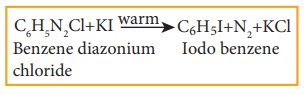
3) Preparation of iodobenzene
Iodobenzene
is prepared by warming benzene diazonium chloride with aqueous KI solution.
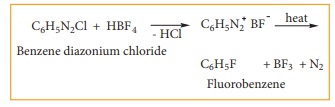
4) Preparation of fluorobenzene
Fluoro
benzene is prepared by treating benzenediazonium chloride with fluoro boric
acid. This reaction produces diazonium fluoroborate which on heating produces
fluorobenzene. This reaction is called Balz – schiemann reaction.
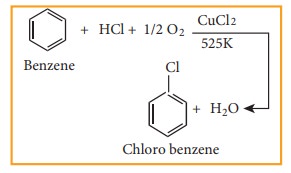
5) Commercial preparation of chloro benzene (Raschig process)
Chloro
benzene is commercially prepared by passing a mixture of benzene vapour, air
and HCl over heated cupric chloride .This reaction is called Raschig process.
Physical properties
1. Melting and boiling points
The
boiling points of monohalo benzene which are all liquids follow the order
Iodo
> Bromo > Chloro
The
boiling points of isomeric dihalobenzene are nearly the same
The
melting point of para isomer is generally higher than the melting points of
ortho and meta isomers. The higher melting point of p-isomer is due to its
symmetry which leads to more close packing of its molecules in the crystal
lattice and consequently strong intermolecular attractive force which requires
more energy for melting
p
–Dihalo benzene > o- Dichloro benzene > m-Dichloro benzene
2. Solubility
Haloarenes
are insoluble in water because they cannot form hydrogen bonds with water ,but
are soluble in organic solvents
3. Density
Halo
arenes are all heavier than water and their densities follow the order.
Iodo
benzene > Bromo benzene > Chloro benzene
Chemical properties
A. Reactions invoving halogen atom
1. Aromatic nucleophilic substitution reaction
Halo
arenes do not undergo nucleophilic substitution reaction readily. This is due
to C–X bond in aryl halide is short and strong and also the aromatic ring is a
centre of high electron density.
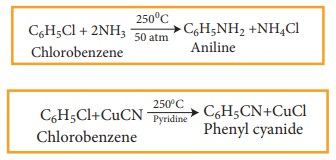
The
halogen of haloarenes can be substituted by OH– , NH2–,
or CN– with appropriate nucleophilic reagents at high temperature
and pressure.
For Example
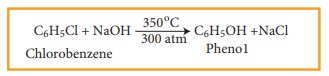
This
reaction is known as Dow’s Process
2. Reaction with metals
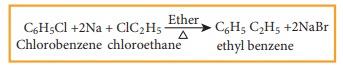
a) Wurtz Fittig reaction
Halo
arenes reacts with halo alkanes when heated with sodium in ether solution to
form alkyl benzene. This reaction is called wurtz fittig reaction.
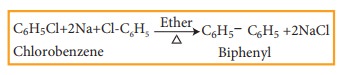
b) Fittig reaction
Haloarenes
react with sodium metal in dry ether, two aryl groups combine to give biaryl
products. This reaction is called fittig reaction
B) Reaction involving aromatic ring
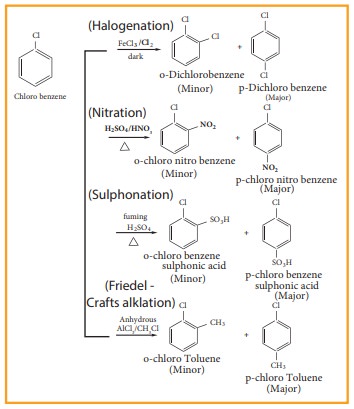
3. Electrophilic substitution reaction
Haloarenes
undergo aromatic electrophilic substitution reactions. The rate of eleclophilic
substitution of halobenzene is lower than that of benzene. halogen is
deactivating due to - I effect of halogen. The lone pair of electrons on the
chlorine involves in resonance with the ring. It increases the electron density
at ortho and para position (refer figure no 14.1). The halogen attached to the
benzine ring with draw electron and thereby and hence the halogen which is
attached to the benzene directs the incoming, electrophile either to ortho or
to para position in electrophilie substitution reaction
Toluenes.
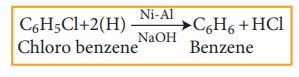
4) Reduction
Haloarenes
on reduction with Ni-Al alloy in the presence of NaOH gives corresponding
arenes.
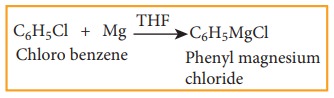
5) Formation of Grignard reagent
Haloarenes
reacts with magnesium to form Grignard reagent in tetra hydrofuran (THF).
Uses of Chloro benzene
i.
Chloro benzene is
used in the manufacture of pesticides like DDT
ii.
It is used as high boiling solvent in organic synthesis.
iii.
It is used as fibre - swelling agent in textile processing.
Related Topics