Nomenclature and isomerism, Preparation, Physical and Chemical properties, Mechanism, Uses, IUPAC name, structural formula | Chemistry - Haloalkanes | 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Haloalkanes
Haloalkanes
Mono
halogen derivatives of alkanes are called haloalkanes. Haloalkanes are
represented by general formula R ŌĆō X, Where, R is an alkyl group (CnH2n+1)
ŌĆō and X is a halogen atom (X=F, Cl, Br or I). Haloalkanes are further
classified into primary, secondary, tertiary haloalkane on the basis of type of
carbon atom to which the halogen is attached.
Primary haloalkane
Examples:
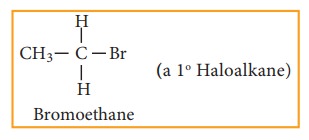
Secondary haloalkane
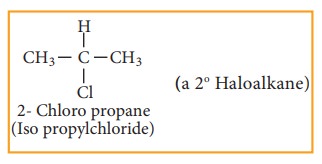
Tertiary haloalkane
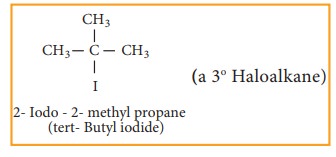
Nomenclature
Common system
In
the common system, haloalkanes are named as alkyl halides. It is derived by
naming the alkyl group followed by the halide.
IUPAC system
Let
us write the IUPAC name for the below mentioned haloalkanes by applying the
general rules of nomeclature that are already discussed in Unit no : 11

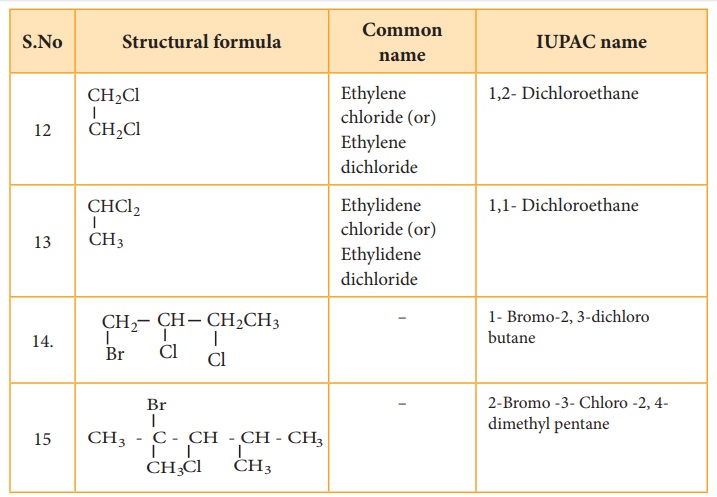
Poly halogen Compounds:
The common and IUPAC name of polyhalogen compounds are give below
Nature of C ŌĆō X bond in haloalkane
Carbon
halogen bond is a polar bond as halogens are more electro negative than carbon.
The carbon atom exhibits a partial positive charge (ą▒+) and halogen atom a partial
negative charge (ą▒-)
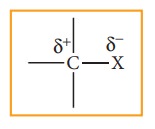
The
C ŌĆōX bond is formed by overlap of sp3 orbital of carbon atom with
half filled p- orbital of the halogen atom. The atomic size of halogen
increases from fluorine to iodine, which increases the C ŌĆō X bond length.
Larger the size, greater is the bond length, and weaker is the bond formed. The
bond strength of C ŌĆō X decreases from C ŌĆō F to C ŌĆō I in CH3X. The
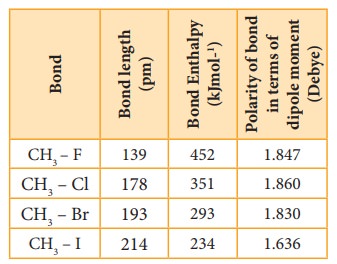
changes in the value of bond length, bond enthalpy and bond polarity, as we
more from C ŌĆōF to C ŌĆōI, is given in the table.
Table showing carbon ŌĆō halogen bond length, bond enthalpy and polarity of bond.
Methods of preparation
Haloalkanes
are prepared by the following methods
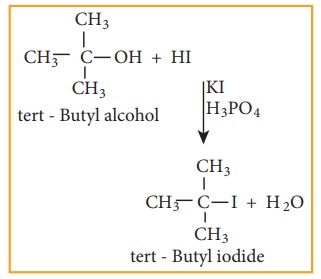
1) From alcohols
Alcohols can be converted into halo alkenes by reacting it with any one of the following reagent 1. hydrogen halide 2. Phosphorous halides 3. Thionyl chloride
a) Reaction with hydrogen halide
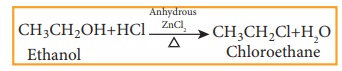
Mixture
of con.HCl and anhydrous ZnCl2 is called Lucas reagent.
The
order of reactivity of halo acids with alcohol is in the order HI > HBr >
HCl. The order of reactivity of alcohols with halo acid is tertiary >
secondary > primary.
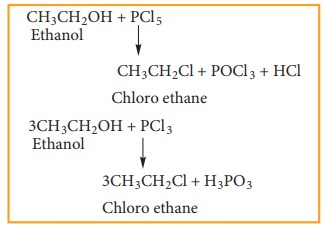
b) Reaction with phosphorous halides
Alcohols
react with PX5 or PX3 to form haloalkane. PBr3
and PI 3 are usually generated in situ (produced in the reaction
mixture) by the reaction of red phosphorus with bromine and iodine,
respectively.
Example
c) Reaction with thionyl chloride (Sulphonyl chloride)
Example
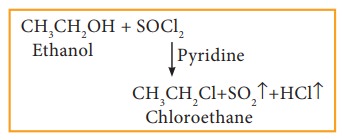
This
reaction is known as Darzen's halogenation
2) From alkenes
Alkenes
react with halogen acids (HCl, HBr, HI) to give haloalkane. The mode of
addition follows MarkovnikovŌĆÖs rule.
3) From alkanes
Alkanes
react with halogens (Cl2 or Br2) in the presence of ultra
violet light to form haloalkane. This reaction is a free radical substitution
reaction and gives a mixture of mono, di or poly substituted haloalkane.
Example
Chlorination
of methane gives different products which have differences in the boiling
points. Hence, these can be separated by fractional distillation.
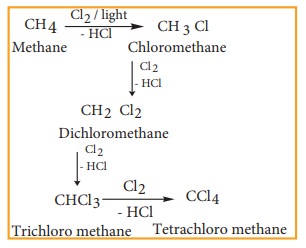
4) Halogen exchange reactions
a) Finkelstein reaction
Chloro
or bromoalkane on heating with a concentrated solution of sodium iodide in dry
acetone gives iodo alkanes. This reaction is called Finkelstein reaction, (SN2
reaction).
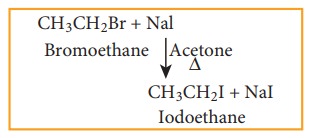
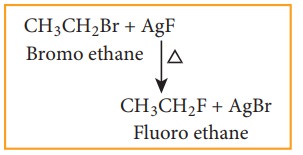
b) Swarts reaction
Chloro
or bromo alkanes on heating with metallic fluorides like AgF, SbF3
or Hg2F2 gives fluoro alkanes. This reactions is called
Swarts reaction.
Example
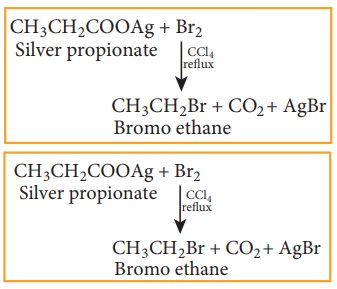
5) From silver salts of fatty acids (Hunsdiccker reaction)
Silver
salts of fatty acids when refluxed with bromine in CCl4 gives bromo
alkane
Physical Properties
1.
Pure haloalkanes are colourless. Bromo and iodo alkanes are coloured in the
presence of light.
2.
Haloalkanes having one, two or three carbon atoms are in the gaseous state at
normal temperature. Haloalkanes having more than three carbon atoms are liquids
or solids.
3). Boiling point and Melting point
i)
Haloalkanes have higher boiling point and melting point than the parent alkanes
having the same number of carbons because the intermolecular forces of
attraction (dipole ŌĆō dipole interaction and vander Waals forces) are stronger in
haloalkane.
ii)
The boiling point and melting point of haloalkanes decreases with respect to
the helogen in the following order.
Example
CH3I
> CH3Br > CH3Cl > CH3F
iii)
The boiling points of chloro, bromo and iodo alkanes increase with the increase
in the number of halogen atoms.
For Example:
CCl4 > CHCl3 > CH2Cl2 > CH3Cl
iv)
The boiling point and melting point of mono haloalkane increase with the
increase in the number of carbon atoms.
Example
CH3CH2CH2Cl
> CH3CH2Cl > CH3Cl
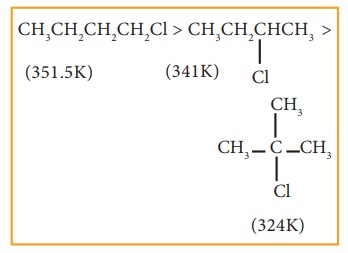
v)
Among isomeric alkyl halides the boiling point decreases with the increase in
branching in the alkyl group; with increase in branching, the molecule attains
spherical shape with less surface area. As a result the inter molecular forces
become weak, resulting in lower boiling points.
Example
4. Solubility
Haloalkanes are polar covalent compounds soluble in organic solvents, but insoluble in water because they cannot form hydrogen bonds with water molecules
5. Density
The density of liquid alkyl halides are higher than these of hydrocarbons of comparable molecular weight.
Chemical properties
Haloalkanes
are one of the most reactive classes of organic compounds. Their reactivity is
due to the presence of polar carbon ŌĆō halogen bond in their molecules. The
reactions of haloalkane may be divided into the following types
1.
Nucleophilic substitution reactions
2.
Elimination reactions
3.
Reaction with metals
4.
Reduction
1) Nucleophilic substitution reactions
We
know that the C╬┤+ - X╬┤- present in halo alkane is polar
and hence the nucleophilic reagents are attracted by partially positively
charged carbon atoms resulting in substitution reactions.
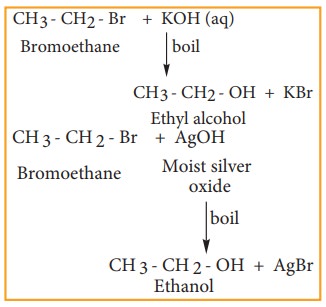
Reaction with aqueous alkali or moist silver oxide.(Hydrolysis)
Haloalkane
reacts with aqueous solution of KOH or moist silver oxide (Ag2O/H2O)
to form alcohols.
Example
i) Reaction with alcoholic ammonia (Ammonolysis)
Haloalkanes
react with alcoholic ammonia solution to form alkyl amines.
Example
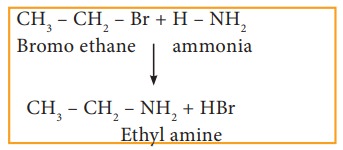
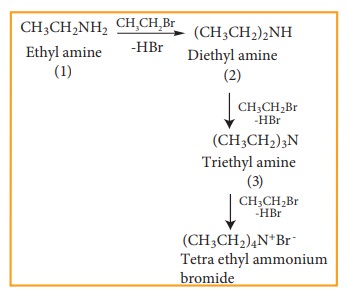
However,
with excess of halo alkane, secondary and tertiary amines along with quartenary
ammonium salts are obtained
Ambident Nucleophiles
Nucleophiles
such as cyanide and nitrite ion which can attack nucleophilic centre from two
sides are called ambident nucleophiles. These nucleophiles can attack with
either of the two sides depending upon the reaction conditions and the reagent
used.
ii) Reaction with alcoholic KCN
Haloalkanes
react with alcoholic KCN solution to form alkyl cyanides.
Example
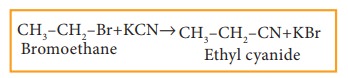
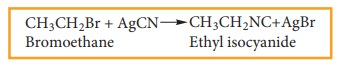
iii) Reaction with alcoholic AgCN
Haloalkanes
react with alcoholic AgCN solution to form alkyl isocyanide.
Example
iv) Reaction with sodium or potassi-um nitrite
Haloalkanes
react with alcoholic solution of NaNO2 or KNO2 to form
alkyl nitrites.
Example
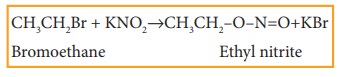
v) Reaction with silver nitrite
Haloalkanes react
with alcoholic solution of AgNO2
to form nitro alkanes. Example
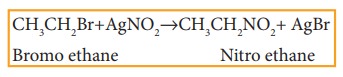
vi) Reaction with sodium or potassi-um hydrogen sulphide
Haloalkanes
react with sodium or potassium hydrogen sulphide to form thio alcohols.
Example
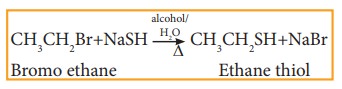
vii) Williamson ether synthesis
Haloalkane,
when boiled with sodium alkoxide gives corresponding ethers.
Example
This
method can be used to prepare mixed (unsymmetrical) ethers also.
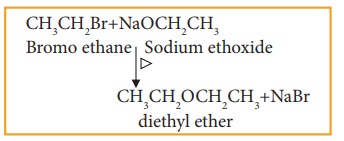
Mechanism of Nucleophilic substitu-tion reaction
The
mechanism of nucleophilic substitution reaction is classified as
a) Bimolecular Nucleophilic substitution reaction (SN2)
b) Unimolecular Nucleophilic substitution reaction (SN1)
SN2 Mechanism
The
rate of SN2 reaction depends upon the concentration of both alkyl
halide and the nucleophile.
Rate
of reaction = k2 [alkylhalide] [nucleophile]
It
follows second order kinetics and occurs in one step.
This
reaction involves the formation of a transition state in which both the
reactant molecules are partially bonded to each other. The attack of
nucleophile occurs from the back side (i.e opposite to the side in which the
halogen is attacked). The carbon at which substitution occurs has inverted
configuration during the course of reaction just as an umbrella has tendency to
invert in a wind storm. This inversion of configuration is called Walden
inversion; after paul walden who first discovered the inversion of
configuration of a compound in SN2 reaction.
SN2
reaction of an optically active haloalkane is always accompanied by inversion
of configuration at the asymmetric centre. Let us consider the following
example
When 2 - Bromooctane is heated with sodium hydroxide, 2 ŌĆō octanol is formed with invesion of configuration. (-) ŌĆō 2 ŌĆō Bromo octane is heated with sodium hydroxide (+) ŌĆō 2 ŌĆō Octanol is formed in which ŌĆō OH group occupies a position opposite to what bromine had occupied,
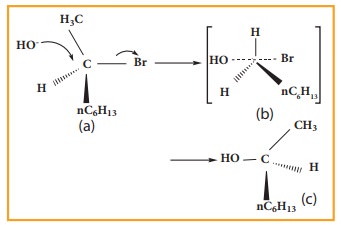
a)
(-) 2 ŌĆō Bromo octane
b)
Transition State
c)
(+) 2 ŌĆō Octanol (product)
SN1 Mechanism
SN1
stands for unimolecular nucleophilic substitution
ŌĆśSŌĆÖ
stands for substitution
ŌĆśNŌĆÖ
stands for nucleophilic
ŌĆś1ŌĆÖ
stands for unimolecular (one molecule is involved in the rate determining step)
The
rate of the following SN1 reaction depends upon the concentration of
alkyl halide (RX) and is independent of the concentration of the nucleophile
(OHŌłÆ).
Hence Rate of the reaction = k[alkyl halide]
RŌłÆCl
+ OHŌłÆ ŌåÆ R ŌĆō OH + ClŌłÆ
This
SN1 reaction follows first order kinetics and occurs in two steps.
We
understand SN1 reaction mechanism by taking a reaction between
tertiary butyl bromide with aqueous KOH.
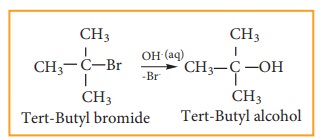
This
reaction takes place in two steps as shown below
Step - 1 Formation of
carbocation
The
polar C - Br bond breaks forming a carbocation and bromide ion. This step is
slow and hence it is the rate determining step.
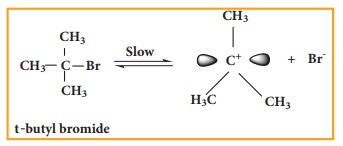
The
carbocation has 2 equivalent lobes of the vacant 2p orbital, so it can react
equally rapidly from either face
Step - 2
The
nucleophile immediately reacts with the carbocation. This step is fast and
hence does not affect the rate of the reactions.
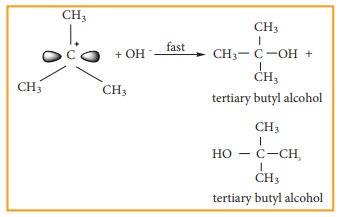
As
shown above, the nucleophilic reagent OH- can attack carbocation from both the
sides.
![]()
![]() In the above example the substrate tert-butyl bromide is not
optically active, hence the obtained product is optically inactive. If halo
alkane substrate is optically active then, the product obtained will be
optically inactive racemic mixture. As nucleophilic reagent OH- can attack
carbocation from both the sides, to form equal proportion of dextro and
levorotatory optically active isomers which results in optically inactive
racemic mixture.
In the above example the substrate tert-butyl bromide is not
optically active, hence the obtained product is optically inactive. If halo
alkane substrate is optically active then, the product obtained will be
optically inactive racemic mixture. As nucleophilic reagent OH- can attack
carbocation from both the sides, to form equal proportion of dextro and
levorotatory optically active isomers which results in optically inactive
racemic mixture.
Example
Hydrolysis
of optically active 2 - bromo butane gives racemic mixture of ┬▒ butan-2-ol
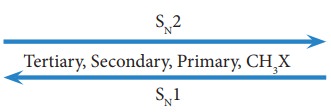
The
order of reactivity of haloalkanes towards SN1 and SN2
reaction is given below. SN2 reaction
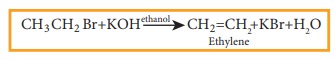
2) Elimination reactions
When
a haloalkene containing a hydrogen on ╬▓ carbon is treated with an ethanolic
solution of potassium hydroxide, an alkene is formed. In this reaction a double
bond between ╬▒ and ╬▓ carbon is formed by releasing a halogen attached to a ╬▒
carbon and a hydrogen to a ╬▓ carbon of halo alkane. This reaction is called ╬▓
elimination reaction. (dehydrohalogenation).
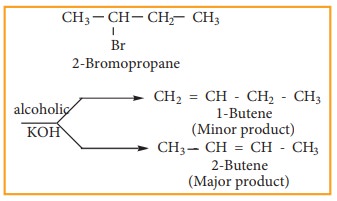
Some
haloalkanes yield a mixture of olefins in different amounts. It is explained by
SaytzeffŌĆÖs Rule, which states that ŌĆśIn a dehydrohalogenation reaction, the preferred
product is that alkene which has more number of alkyl groups attached to the
doubly bonded carbon (more substituted double bond is formed)
Example
Elimination
reactions may proceed through two different mechanisms namely E1 and
E2
E2 reaction mechanism

The
rate of E2 reaction depends on the concentration of alkyl halide and
base
Rate
= k [alkyl halide] [base]
It
is therefore, a second order reaction. Generally primary alkyl halide undergoes
this reaction in the presence of alcoholic KOH. It is a one step process in
which the abstraction of the proton from the ╬▓ carbon and expulsion of halide
from the ŌłØ carbon occur simultaneously. The
mechanism is shown below.
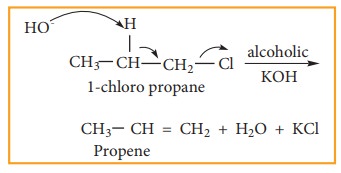
E1
reaction mechanism

Generally,
tertiary alkyl halide which undergoes elimination reaction by this mechanism in
the presence of alcoholic KOH. It follows first order kinetics. Let us consider
the following elimination reaction.
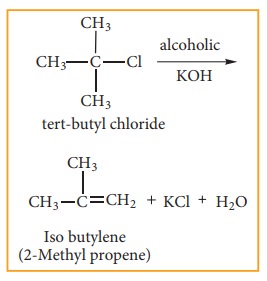
Step - 1 Heterolytic fission to
yield a carbo-cation
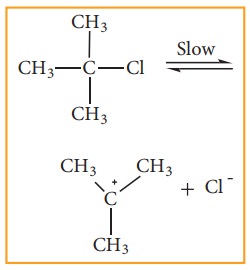
Step - 2 Elimination of a proton
from the - carbon
to produce an alkene
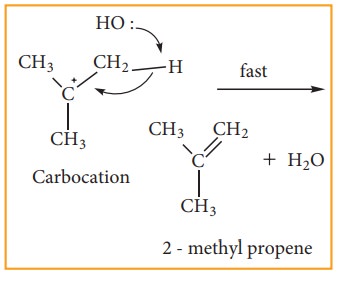
3) Reaction with metals
Haloalkane
reacts with metals, to form a compound containing carbon - metal bond known as
organometallic compounds.
a) Grignard reaction
When
a solution of halo alkane in ether is treated with magnesium, we get alkyl
magnesium halide known as Grignard reagent.
Example
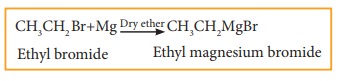
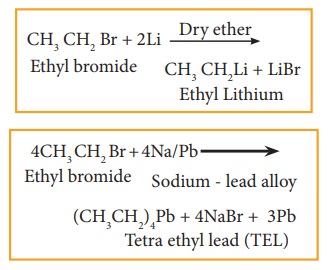
b) Reaction with active metals like so-dium, lead etc
Haloalkane reacts with active metals like sodium, lead etc
in the presence of dry ether to form organo metallic compounds.
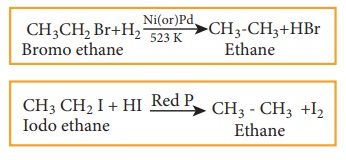
4) Reduction reactions
Haloalkanes
are reduced to alkanes by treating with H2 in the presence of metal
catalyst like nickel, palladium etc or with hydroiodic acid in the presence of
red phosphorous.
The
chemistry of haloalkane can be well understood by the following flowchart.
Uses of haloalkane
Chloroform: As a solvent in pharmaceutical industry and for producing pesticides
and drugs As an anaesthetic.
As
a preservative for anatomical specimens.
Iodoform: Iodoform is used as an antiseptic for dressing wounds.
Carbon tetrachloride: Carbon tetrachloride is used as dry cleaning agent
It
is used as a solvent for oils, fats and waxes
As the vapour of CCl4 is non ŌĆō combustible, it is used under the name pyrene for extinguishing the fire in oil or petrol.
Related Topics