Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Osmosis in solution
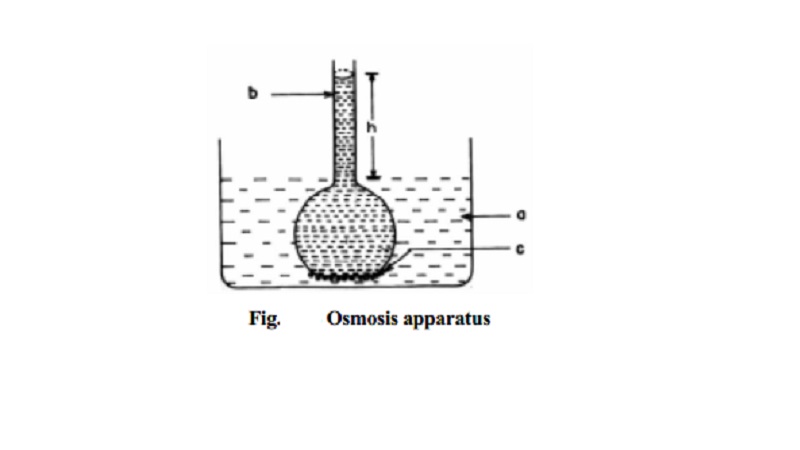
Osmosis in solution
Spontaneous movement of solvent particles from a dilute solution or from
a pure solvent towards the concentrated solution through a semipermeable
membrane is known as osmosis (Greek
word : 'Osmos' = to push).
Fig. depicts Osmosis in a simple way. The flow
of the solvent from its side (a) to solution side (b) separated by
semipermeable membrane
(c) can be stopped if some definite extra pressure is applied on the
solution risen to height (h). This pressure that just stops the flow of solvent
is called osmotic pressure of the
solution. This pressure (p) has been
found to depend on the concentration
of the solution.
Osmosis is a process of prime importance in
living organisms. The salt concentration in blood plasma due to different
species is equivalent to 0.9% of aqueous sodium chloride. If blood cells are
placed in pure water, water molecules rapidly move into the cell. The movement
of water molecules into the cell dilutes the salt content. As a result of this
transfer of water molecules the blood cells swell and burst. Hence, care is
always taken to ensure that solutions that flow into the blood stream have the
same osmotic pressure as that of the blood.
Sodium ion (Na+) and potassium ions
(K+), are responsible for maintaining proper osmotic pressure
balance inside and outside of the cells of organism. Osmosis is also critically
involved in the functioning of kidneys.
Characteristics
of Osmostic Pressure (Pi)
It is the minimum external pressure which must
be applied on solution side in order to prevent osmosis if separated by a
solvent through a semi permeable membrane.
A solution having lower or higher osmotic
pressure than the other is said to be hypotonic or hypertonic respectively in
respect to other solution.
Two solutions of different substances having
same osmotic pressure at same temperature are said to be isotonic to each
other. They are known as isotonic solutions.
Osmotic pressure and
concerned laws
Vant Hoff
noted the striking resemblance between the behaviour of dilute solutions and
gases. He concluded that, a substance in solution behaves exactly like gas and
the osmotic pressure of a dilute solution is equal to the pressure which the
solute would exert if it is a gas at the same temperature occupying the same
volume as the solution. Thus it is proposed that solutions also obey laws
similar to gas laws.
1.Boyle's - Vant Hoff law
The osmotic pressure (Pi) of the solution at
temperature is directly propositional to the concentration (C) of the solution.
Pi Dir. Props to C at constant T
C = Molar concentration
2. Charle's - Vant Hoff
law
At
constant concentration the osmotic pressure(Pi) is directly proportional to the
temperature (T).
Pi
dirc.Prop.to T at constnt C.
Combining
these two laws,
Pi = CRT
Where R is
the gas constant.
Determination of molecular weight by osmotic
pressure measurement
The osmotic pressure is a colligative property
as it depends, on the number of solute molecules and not on their identity.
Solution
of known concentration is prepared by dissolving a known weight (W2)
of solute, in a known volume (V dm3) of the solvent and its osmotic
pressure is measured at room temperature(T).
Since Pi =
CRT
C = n2/ V
= number of moles of solute / Volume of the solution in dm3
C= W2
/ M2V
We get
Pi = W2
R T / M2V
M2 =
W2 R T / Pi V
Thus M2, molecular weight of the
solute can be calculated by measuring osmotic pressure value.
Determination of
osmotic pressure by Berkley-Hartley method
The osmotic pressure of a solution can be
conveniently measured by Berkley - Hartley method. The apparatus (Fig.)
consists of two concentric tubes. The inner tube (a) is made of semipermeable
membrane
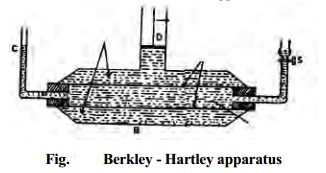
(c) with
two side tubes. The outer tube (b) is made of gun metal which contains the
solution. The solvent is taken in the inner tube. As a result of osmosis, there
is fall of level in the capillary indicator (d) attached to the inner tube. The
external pressure is applied by means of a piston (e) attached to the outer
tube so that the level in the capillary indicator remains stationary at (d).
This pressure is equal to the osmotic pressure (p) and the solvent flow from inner to outer tube is also stopped.
Advantages of this
Method
The osmotic pressure is recorded directly and
the method is quick.
There is no change in the concentration of the
solution during the measurement of osmotic pressure.
The osmotic pressure is balanced by the external pressure and there is minimum strain on the semipermeable membrane.
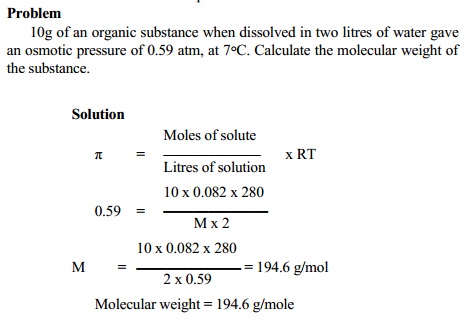
Related Topics