Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Numerical values of gas constant (R)
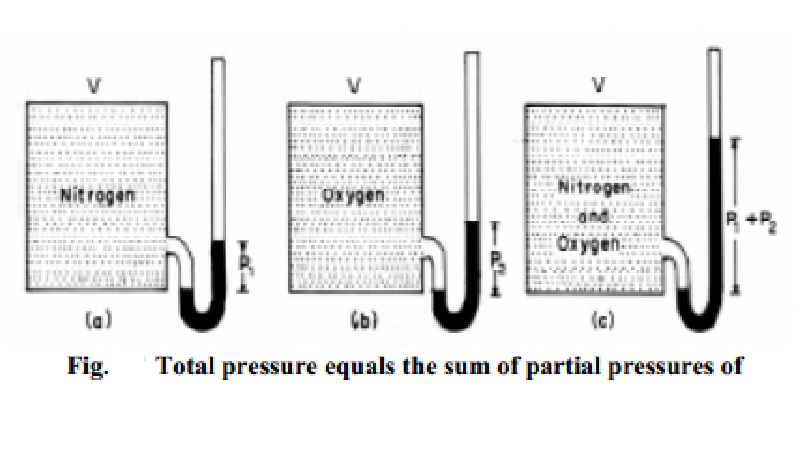
Numerical values of
gas constant (R)
The
numerical value of the gas constant `R' depends upon the units in which
pressure and volume are expressed,
R
= PV / T (assuming one mole of gas)
R =
(Pressure x Volume) / Temperature
=
( Force / Area ) x ( Volume / Temperature )
Since Volume = Area x length
R
= ( Force x Length ) / Temperature
=
Work / Temperature
The dimensions of R are thus energy per degree per mole.
i) In litre - atmosphere
One mole
of gas at S.T.P occupies a volume of 22.4 litre and thus
At STP
P = 1 atm R = PV/T (for 1 mole)
V = 22.4 litre R = (1 x 22.4)/273
T = 273 K R = 0.0821
litre atm K-1mol-1
= 0.0821 dm3.atm K-1mol-1
(ì 1 litre =
1 dm3)
ii) In C.G.S. System
At STP 1 mole of gas has
P = 1 atm = 1 x 76 x 13.6 x 980 dyne cm-2
= 1.013 x 106 dyne cm-2
V = 22400 cm3; T = 273 K
R = PV / T = 1.013 x 106
x 22400 / 273
R = 8.314 x 107 erg K-1 mol-1
iii) In M.K.S. System
In MKS or SI units, the unit of R is joule
Since 107
erg = 1 Joule
R = 8.314 Joule K-1 mol-1
1 calorie = 4.184 Joule
R = 1.987 cals deg-1 mol-1
Related Topics