Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Nitrogen Group
Nitrogen Group
The
elements nitrogen, phosphorus, arsenic, antimony and bismuth constitute 15th
group of the periodic table. This group is called nitrogen group. These
elements have the general electronic configuration ns2np3.
All these elements have five electrons in their outermost orbitals. The `s'
orbital contains two electrons and p orbital contains three electrons. These
three electrons are equally distributed in three p-orbitals as px1
py1 pz1 which correspond to half-filled configuration.
As we go down the group, the two electrons
present in the valence `s' orbital become inert and only the three electrons
present in the outermost p-orbitals are involved in chemical combinations. This
is known as inert pair effect. As we move from nitrogen to bismuth, the
pentavalency becomes less pronounced while trivalency becomes more pronounced.
Nitrogen was discovered in 1772 by Daniel
Rutherford, a Scottish physician and chemist. Elementary nitrogen constitutes
three-fourths of air by weight. It is also abundant in the combined state as
saltpetre (KNO3), sodium nitrate (chile saltpetre) and ammonium
salts. Nitrogen is an essential constituent of all vegetable and animal
proteins.
Fixation of nitrogen
The nitrogen present in the atmosphere is free
or elementary nitrogen whereas nitrogen present in various nitrogenous
compounds is called combined or fixed nitrogen. The conversion of free atmospheric
nitrogen to a nitrogen compound is called fixation of nitrogen.
Method employed for fixation or bringing
atmospheric nitrogen into combination:
Manufacture of ammonia (Haber's process) :
A mixture of nitrogen and hydrogen in the ratio
1:3 under pressure (200-900 atm) is passed over a catalyst finely divided iron
and molybdenum as promoter, heated to about 770K.
N2 + 3H2 -- > < -- 2NH3
The
ammonia so manufactured can be xidized to nitric oxide by passing a mixture of
ammonia and air over heated platinum gauze at 1070K. Nitric oxide combines with
more of oxygen to give nitrogen dioxide which when absorbed in water in the
presence of excess of air, gives nitric acid (Ostwald's process).
4NH3 + 5O2 -- > 4NO + 6H2O
2NO + O2
-- > 2NO2
4NO2 + 2H2O
+ O2 -- > 4HNO3
Ammonia and nitric acid manufactured above may be converted into
ammonium salts and nitrates suitable as fertilizers. Thus these methods of
nitrogen fixation are of vital importance to the agriculturists.
Nitrogen fixation in nature
Due to electrical disturbances atmospheric nitrogen and oxygen combine
to give nitric oxide which gets further xidized to nitrogen dioxide. This
reacts with rain water in the presence of excess of oxygen to produce nitric
acid and is washed down to earth. Here it reacts with bases of the soil to give
nitrates.
In addition to this, certain bacteria living in the nodules on roots of
leguminous plants e.g. pea, beans etc., convert nitrogen into nitrogenous
compounds which can be directly assimilated by the plant.
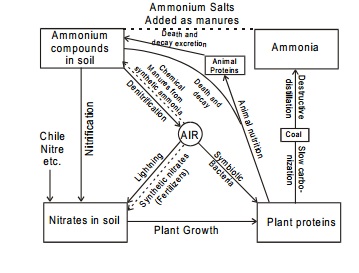
Nitrogen cycle
There is a
continual turnover of nitrogen between the atmosphere, the soil, the sea and
living organisms. The nitrogen passes from atmosphere to plants and animals,
converted into useful products like ammonia, nitric acid etc. and still its
percentage in the atmosphere remains practically unchanged. This is due to the
fact that combined nitrogen is constantly passing back to the atmosphere. This
cycle of changes involved is known as nitrogen cycle.
Uses of nitrogen
compounds
1.
Liquid ammonia is used as solvent.
2.
Ammonia is used as a refrigerant in ice-plants.
3.
Ammonia is used in the manufacture of artificial
silk, urea, manures, washing soda etc.
4.
Nitrous oxide mixed with oxygen is used as
anaesthetic for minor operations in dentistry and surgery.
5.
Nitrous acid is used in the manufacture of azo-dyes.
6.
Nitric acid is used in the manufacture of
fertilizers, explosives like TNT, GTN, etc.
7.
Nitric acid is used in the purification of gold
and silver.
8.
Nitric acid is used in pickling of stainless
steel.
9.
Nitric acid is used in the manufacture of perfumes,
artificial silk, medicines etc.
10.
Liquid nitrogen is used as a refrigerant.
Nitric acid
Nitric acid is an important oxyacid of nitrogen.
It was called as `aqua tortis' by alchemists. It means strong water. It was
first prepared by Glauber (1650). Later Cavendish (1784) stated that nitric
acid may be formed by passing electric sparks through the mixture of nitrogen
and moist oxygen. Traces of nitric acid occur in air where it is formed by
electric sparks through the mixture of nitrogen and moist oxygen. Traces of
nitric acid occur in air where it is formed by electric discharges and is
washed down by rain.
Preparation
1. Laboratory preparation
Nitric acid is prepared in the laboratory by heating a nitrate salt with
concentrated sulphuric acid.
NaNO3 + H2SO4 -- > NaHSO4 + HNO3
Vapours of nitric acid are condensed to a brown liquid in a receiver
cooled under cold water. Dissolved oxides of nitrogen are removed by
redistillation or blowing a current of carbondioxide or dry air through the
warm acid.
2. Manufacture of nitric
acid
Nitric acid is manufactured by blowing air into an electric arc struck
between two water cooled copper electrodes and spread into a disc with the help
of a magnetic field at right angle. The serious disadvantage of the method is
now obsolete.
3. Ostwald's process
Large
quantities of ammonia manufactured by Haber's process are converted into nitric
acid by Ostwald's process.
4 NH3 - (Platinum
gauze) -- > 4NO + 6H2O
2NO + O2
-- > 2 NO2
4NO2 + 2H2O + O2 -- > 4 HNO3
Dilute nitric acid may be concentrated by distillation until a constant
boiling point mixture is obtained (98%). Fuming nitric acid is obtained by
distilling this acid with concentrated sulphuric acid. Crystals of pure nitric
acid may be obtained by cooling 98% acid in a freezing mixture.
Properties
Physical properties
1.
It is a colourless fuming liquid when pure, but
may be coloured yellow by its dissociation products mainly nitrogen dioxide.
2.
It has extremely corrosive action on the skin
and causes painful sores.
3.
Pure acid has a specific gravity of 1.54. It
boils at 359K and freezes to a white solid (m.p. 231K).
Related Topics