Chapter: 11th Chemistry : UNIT 14 : Haloalkanes and Haloarenes
Nature of C – X bond in haloalkane
Nature of C – X bond in haloalkane
Carbon halogen bond is a polar bond as halogens are more electro negative than carbon. The carbon atom exhibits a partial positive charge (б+) and halogen atom a partial negative charge (б-)
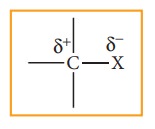
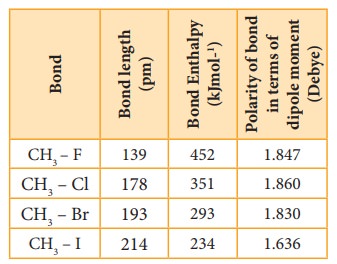
The C –X bond is formed by overlap of sp3 orbital of carbon atom with half filled p- orbital of the halogen atom. The atomic size of halogen increases from fluorine to iodine, which increases the C – X bond length. Larger the size, greater is the bond length, and weaker is the bond formed. The bond strength of C – X decreases from C – F to C – I in CH3X. The changes in the value of bond length, bond enthalpy and bond polarity, as we more from C –F to C –I, is given in the table.
Table showing carbon – halogen bond length, bond enthalpy and polarity of bond.
Related Topics