Chapter: Aquaculture Principles and Practices: Nutrition and Feeds
Live animal food: Nature and source of live foods
Live animal food
Larval and adult stages of many aquaculture species grow well on live animal foods, especially zooplanktonic organisms. The two major sources for such organisms are collections from natural waters or culture under controlled conditions. The production of plankton in ponds and other impoundments by organic and inorganic fertilization will be discussed.
Besides the small-scale collection of plankton from natural waters using standard or modified plankton nets, large-scale plankton fishing is done in certain areas. Propeller pumps and net cones have been used successfully for fishing in the sea in Norway. Two net cones, one inside the other, are used for filtering. The outside cone has a smaller mesh (about 250 mm). Plankton goes through the inner cone, but is retained by the outer one. A hose from the tip of the outer cone leads the plankton to the larvae-rearing units which, as far as possible, are located nearby.Another method is based on
the phototactic reaction of many plankton species. They are attracted by underwater lights directly into net cages containing larvae to be fed, or pumped away by an air lift. Fishing for plankton from natural waters may be more eco-nomical, but obviously there are many uncertainties in the quality and quantity of plankton that can be obtained. So in any large-scale aquaculture, particularly in hatchery production of larvae, culturing plankton will be more dependable.
Brine shrimp
Among live animal foods used in aquaculture, the brine shrimp Artemia salina has received considerable attention in recent years, largely due to its expanding use in crustacean hatcheries. In most cases, Artemia is used as freshly hatched or frozen nauplii, starting from dry cysts. Dry cysts are commercially available and sources of supply have increased. Most of the Artemia cysts are collected from accumulations along the shores of salt lakes and as a by-product of salt production. Until recently, the commercial exploitation of Artemia has been restricted to only a few places. Because of increasing demand and high market prices, Artemia has now been transplanted into newenvironments where they have started yielding appreciable quantities of cysts.
Nutritionally, there may be better live animal food for larvae than Artemia, but the ease and speed with which the cysts can be hatched make their use very convenient in hatcheries. Studies have shown that nauplii from cysts from different geographical regions do not give equally good results in larval rearing. Differences between strains, modes of harvesting, handling and hatching may all affect their nutritive values. Cysts that accumulate on the shores of salt lakes may be subjected to repeated hydration and dehydration due to rainfall and other atmospheric conditionsbefore they are collected, which may take up to several weeks. The size of nauplii from such cysts and their energy contents may therefore be below normal. It has also been observed that transplanted Artemia have reduced cyst production. Solar salt works and ponds in wet tropics have been used for full-cycle Artemia culture, but the economics of combining it with commercial salt production has not yet been established. It is thought that the evaporation ponds of salt works can be used simultaneously for Artemia production.
Because of the variations in hatching rate, length and weight of nauplii and nutrient contents, the cysts have to be selected carefully. The size of nauplii that the larvae can ingest is also an important factor in selecting the strain. It is reported that more than 50 strains have been registered. Liao et al. (1983) have compiled data on the performance of cysts from different sources (Table 7.13), which could provide some guidance in the selection of sources of cysts.
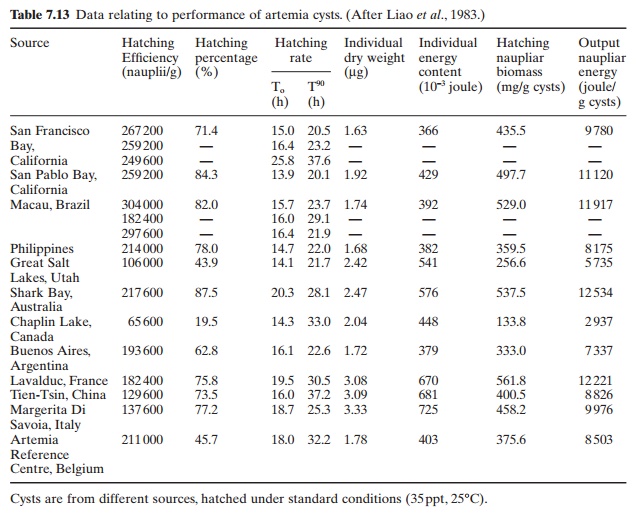
Hatching efficiency and hatching percentage are probably the most important characteristics, but a shorter hatching rate ensures better cysts and greater reliability of supplies of nauplii on a timely basis. Also, heavier individual weights and a greater hatching output provide higher nutritional values.
Hatching of Artemia cysts and production of nauplii for feeding larvae in aquaculture farms are relatively easy. Most types of hatchery tanks can be used for the purpose. Some aquaculturists prefer cylindroconical containers, with water circulation to keep the cysts in suspension; others recommend funnel-shaped containers including heat-sealed plastic bags that are aerated from the bottom. As far as possible, natural sea water with a temperature
of 25–30°C and pH of 8–9 should be used. High dissolved oxygen contents up to saturation should be maintained in the tanks. For optimal results, a continuous illumination of about 1000 lux should be maintained. This can be accomplished in a 75 l tank by suspending two 60 W fluorescent light bulbs at a distance of about 20 cm. A density of less than 10 g cysts per l water is recommended for hatching.The nauplii should be harvested soon after hatching, for feeding larvae of crustaceans or fish, in the instar I stage when they have the highest calorific content. After the second moulting, that generally takes place within 24 hours after hatching at 25°C, the weight and calorific value of the individual nauplii decreases by over 20 per cent. Harvesting of nauplii can be done by siphoning or by a 125 mm screen net, after an interruption of aeration in the tank. Empty shells float at the surface, while the nauplii concentrate in the lower part of the container. The concentration of nauplii can be increased by utilizing their phototactic properties. The upper part of the container may be covered with a black plastic sheet and the lighting directed to reach the lower part of the tank. To improve the flotation of cyst shells, the salinity of the tank water may be increased up to 35ppt shortly before harvesting, by addition of saturated brine or crude salt (which does not harm the nauplii).
The harvested nauplii should be washed thoroughly on a 125 mm screen prior to feeding to larvae, in order to prevent contamination of larval tanks with glycerol, hatching metabolites and bacteria.
To improve the hatching rate, decapsulation of the cysts is recommended by Sorgeloos et al. (1977). The hard shells of the cysts can be removed by short exposure to a hypochlorite solution. Decapsulation also has the advantage of easy separation of nauplii, disinfection of cysts and a lower threshold for light stimulation at the onset of hatching. The main disadvantage is that extra water circulation with devices such as air/water lifts will be needed in hatching containers, because the cysts have a tendency to settle out of suspension due to the increased buoyancy caused by the loss of chorion.
Decapsulation consists of a series of consecutive treatments which include hydration of the cysts, exposure to hypochlorite solution and washing and deactivation of chlorine residues.
Hydration is done in fresh or sea water not exceeding 35 ppt salinity at a temperature of about 25°C. Full hydration is reached in 1–2 hours, after which the cysts are transferred to a hypochlorite solution. Either liquid bleach NaOCl or bleaching powder Ca(OCl)2 can be used. The weight of active product per volume of the solution required for both of these is the same, namely 0.5 g per g cysts and 14 ml solution per g cysts. The pH of the solution is stabilized by the addition of CaO or Na2CO3 and then aerated for about 10 minutes. It is then stored overnight for precipitation and cooling. The supernatant can be siphoned off the next day for decapsulation.
The same type of containers with an aeration system, as used for the hatching of cysts, can
also be used for decapsulation. The hydrated cysts are transferred to the tank containing the solution and kept in continuous suspension. As the chorions dissolve after exposure to the solution, foam accumulates and a gradual change in the colour of the cysts occurs. When large quantities of cysts (500 g or more) are treated, the temperature of the bath should be monitored, and if it goes above the optimum it should be brought down by the addition of ice. The lethal temperature for the cysts is about 40°C. Complete decapsulation can be achieved in 10–15 minutes, when the cysts should be filtered off and washed well on a 120 mm screen with fresh or sea water, until the smell of chlorine is completely removed.
In order to deactivate toxic residues that may remain adsorbed to the decapsulated cysts, they should then be dipped a couple of times in a bath of chloric or acetic acid. After deactivation in the baths for less than half a minute, the cysts should be washed again with tap or sea water, and will then be ready for incubation. Decapsulated cysts can also be used directly or feeding larvae, but then the volume of the food will be at least 50 per cent less than for hatched nauplii.
Hatched instar stages of nauplii of most strains of Artemia can, if necessary, be stored under refrigeration (0–4°C) in aerated containers for up to 48 hours, with minimal energy losses.
Artemia can be cultivated from nauplius toadult stage under controlled conditions using extensive methods in earthen ponds or intensively, in air/water lift-operated raceway-type tanks (Sorgelos et al., 1983). Some farmers in tropical countries like the Philippines and Brazil have raised adult Artemia in earthen ponds to feed larvae of fish or shrimps. Lime and crude salt are added to sea water to provide an acceptable medium for their culture. Weekly additions of organic and inorganic fertilizers enhance phytoplankton growth to feed the Artemia. Production of more than 10g brineshrimp per square metre per day has been reported.
In the intensive system, raceway-type rectangular or elongated oval tanks with central partitioning and air/water lifts can be used. The height/width ratio of the tank should be close to 1, and the water depth is not allowed to
exceed 1 m to enable optimal water circulation. The central partitioning along the length of the tank leaves enough space at both ends to ensure easy water circulation. Heaters and thermostats can be directly immersed in the culture tank in order to maintain the optimal temperature of 25–30°C. Water evaporation can be reduced by using an insulated cover and the darkness thus created is also conducive to faster growth of Artemia.
Natural sea water enriched with bicarbonate (2 g NaHCO3/l) or artificial sea water is used as the culture medium. The artificial sea water is prepared according to the following recipe:
Evaporated
sea salt 31.08 g/l (tap water)
MgSO4 7.74 g/l (dissolved in hot water)
MgCl2 6.09 g/l
CaCl2 1.53 g/l
KCl 0.97 g/l (dissolved in hot water)
NaHCO3 2.00 g/l (dissolved in hot water)
The cultures are inoculated with freshly hatched nauplii at a rate of about 10 000/l. Several types of small-size feed items that do not dissolve in the culture medium can be used, such as micronized rice bran, spray-dried Spir-ulina, dried algae, yeast, etc. Since Artemia filterfeeds continuously, it is necessary to maintain constant feed densities throughout the day for best results. An automatic food-dispensing device would be most useful. An alternative is to hold aerated suspensions of food in special containers, from which the food is introduced at preset time intervals by an electric clock-activated air pump, which triggers food distribution for preset time periods.
Solid wastes from the tank have to be removed at least every other day from about the fourth day of culture. Dissolved oxygen and the pH are monitored regularly. If oxygen levels drop below 2 mg/l, aeration rates are increased; if the pH drops below 7.5, more NaHCO3 is added.
After two weeks of culturing, the larvae will reach an average length of 8 mm, yielding a wet weight of around 5 kg Artemia per cubic metre of culture media. Harvesting of pre-adults is facilitated by turning off the aeration. When the dissolved oxygen level in the medium drops to critical levels after about 30 minutes, theArtemiaconcentrate at the surface, from wherethey can be scooped out easily.
Much higher quantities of Artemia can be produced in flow-through systems with continuous renewal of culture water and removal of all waste products. This is possible in areas where sufficiently warm sea water or brine can be had inexpensively.
Live Artemia are also a better food for postlarvae of crustaceans and finfish than most artificial feeds. Adult Artemia are omnivorous and can feed on protozoa, microalgae, yeast or bacteria, as well as a variety of artificial feeds. Unused feed materials, when disintegrated, become fertilizers and are recirculated for the production of their natural food items in cultures. Thus the cost of culturing can be greatly reduced.
Pre-adult Artemia are known to be more nutritive than freshly hatched nauplii. During growth, their protein contents increase from about 42 to 60 per cent and the fat contents decrease from about 20 to less than 10 per cent of dry weight. Nauplii are deficient in histidine, methionine, phenylalanine and threonine, but the adults are rich in all amino acids (Tobias et al., 1980). Being an extremely hardy speciesthat can tolerate salinities ranging from 5 to 150 ppt, water temperatures from 6 to 35°C and dissolved oxygen even less than 1 ppm, it is becoming a favoured live food in some countries.
Related Topics