Chapter: Aquaculture Principles and Practices: Nutrition and Feeds
Algal culture - Aquaculture: Nature and source of live foods
Algal culture
The culture of algae for feeding larvae and postlarvae through fertilization and water management has been practised in traditional finfish culture. In recent years there has been greater interest in intensive forms of algal culture, largely because of the need for live foods in rearing larvae in crustacean culture, which has expanded rapidly. Algal culture facilities now form an integral part of many shrimp and prawn hatcheries. Where controlled breeding of molluscs such as oysters is practised, algal culture to feed the larvae is probably the most important activity
Several species of microalgae are cultured for experimental purposes in laboratories or for commercial use in special tanks or batteries of large flasks or carboys. A partial list of commonly cultured algal species is given by Fox (1983).
nutrient levels, nutrient proportions, detention time and mixing required to effect species control in induced blooms of algae. The rapid growth of algae affects the aquatic environment to such an extent that the continued production of those algae is arrested and other species able to grow under the altered environment take over. Despite these limitations, aquaculturists have to use the available techniques to produce the microalgae that appear essential for the culture of larvae or adults of certain species.
The techniques presently employed differ somewhat according to species. But in all cases, an enriched medium is used and the optimum temperature, lighting and aeration are maintained in order to obtain dense growths. Several formulations and variations of media are used and no attempt is made here to describe them all.
Except in laboratory-scale culture, completely pure cultures are not expected. However, efforts are made to maintain as pure a culture of the desired species as necessary and feasible. Where required, it is possible to obtain pure cultures from collections of commercial or non-profit organizations. An alternative would be to isolate them from local collections from natural sources. Isolation is rather complex and difficult but can be done successfully under laboratory conditions. Fox (1983) describes some of the successful techniques employed. All of them involve capillary pippette isolation and the maintenance of stringent aseptic conditions.
For the species mentioned above, the most common culture medium used in mass production is filtered surface seawater enriched with essential growth nutrients. An alternative is a synthetic seawater medium, consisting of distilled water, growth nutrients and artificial sea salts. A variety of inorganic and organic nutrients are used in different types of media. Fox (1983) cites Guillrd’s F/2 medium (Guillard, 1975) (Tabel 7.11) as one that has received extensive use and is suitable for the growth of most algae. The macronutrients in this medium include nitrate, phosphate and silica. Inorganic micronutrients include ferric chloride, the chelate EDTA (to keep essential trace elements in solution) and a number of trace elements. Organic micronutrients include the vitamins thiamin (B1), cyanocobalamin (B12) and biotin. They are not considered essential in all mass cultures of algae. The inclusion of silica is of special importance in the culture of diatoms.
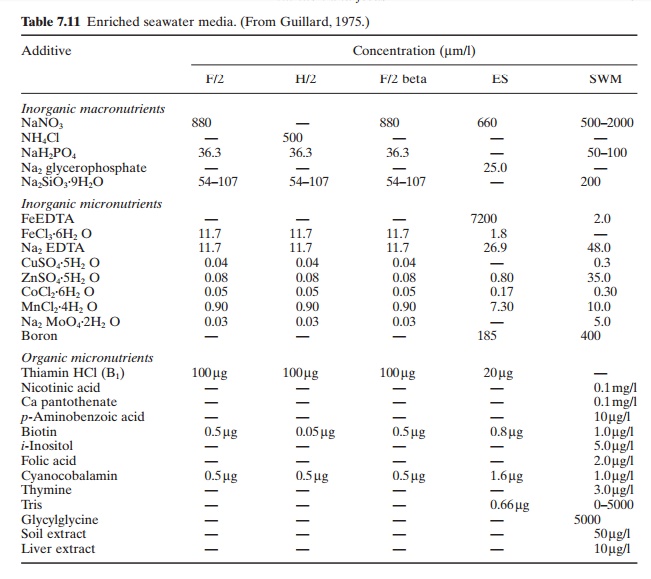
As in the case of enriched seawater media, there are also several formulations of artificial seawater media in use. Fox (1983) cites Gates and Wilson’s NH medium, the composition of which is given in Table 7.12, as a proven medium for the culture of marine algae. Several brands of synthetic sea salts are available in non-sterile powdered form and can be used as an additive; they should be sterilized and homogenized prior to use.
As only small amounts of nutrients are needed at any particular time for algal culture, concentrated nutrient stock solutions are maintained so that the problems of frequently weighing small amounts can be avoided. Not only is time thus saved in preparing media, but the possibility of contamination due to frequent handling of reagents is reduced.
An algal culture system generally consists of three or four main stages, starting with and maintaining a stock culture, from which cultures are made at regular intervals in small flasks (of about 50 ml volume), followed by culture in larger carboys (of about 12 l volume) or tanks of 300 l or more capacity.
Stock cultures can be maintained in small screw-top test tubes that can be autoclaved for sterilization, using low-level enrichment media for maintenance rather than heavy growth.
While constant illumination may be needed for flagellate stocks, a 12-hour photoperiod is considered enough for diatoms. The incident light level required for stock cultures is 750–1000 lux (measured horizontally), which can be provided by two 30–40 W cool white fluorescent bulbs placed in front of the stock culture tubes. A temperature of about 24°C is maintained. After about a month the stock cultures should be transferred under aseptic conditions to create new culture lines.
In the second culture phase, aliquots (2.0 ml) of the stock culture are used to inoculate auto-claved small (about 125 ml) Erlenmeyer flasks. A light intensity of about 1500 lux is necessary. Though aeration may not be necessary, the flasks should be shaken to reduce shading.
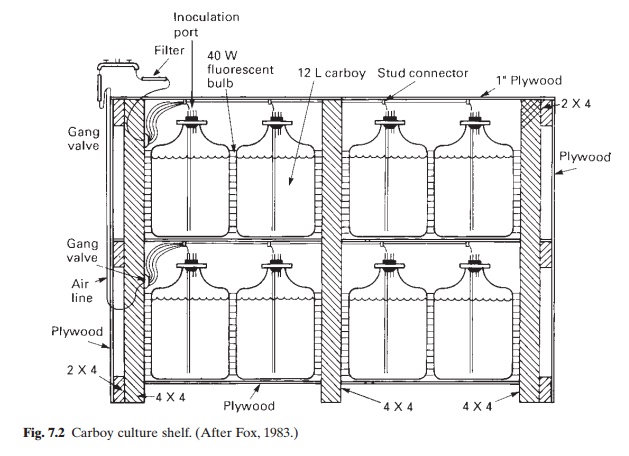
A four-day-old flask culture is used as inoculum for the next phase of culture. Fig. 7.2 illustrates the type and possible arrangements of carboys for algal culture. They are generally 12–20 l in capacity with an autoclaveable stopper fitted with an air supply line and a screw-top inoculation tube. The latter enables more or less aseptic inoculation of cultures and the aeration pipe reduces settling of cells on the bottom, assuring homogeneity of nutrients, exposure to greater light intensity and provision of small amounts of carbon dioxide for growth. A battery of carboys can be arranged on a shelf as shown in fig. 7.2, with large 40 W, cool white fluorescent bulbs placed behind the carboys. Guillard’s F medium, diluted to half strength, is reported to give the high growth rates required for this phase.
After four days of growth in the carboys, the final phase of culture can be started. For this, large fibreglass tanks are used. While for laboratory use 300 l tanks may be suitable, for commercial farms larger tanks of about 1 ton capacity will be necessary. Circular or rectangular tanks, painted white, are often preferred. Illumination is provided by a series of 40 W fluorescent bulbs suspended directly above the tank. Suspended plastic bags have also been successfully used, but would require increased illumination. Constant illumination and aera
Several methods are available for harvesting algae. High-density cultures can be concentrated by chemical flocculation or by centrifuging. Many flocculants cause the cells to settle at the bottom. Others, in combination with an electrical charge, can keep them floating. Harvesting is done by siphoning off the supernatant or by skimming cells off the surface as applicable. Centrifugation of algal cultures can be performed with a standard dairy cream separator. The culture has to be transferred mechanically or by pumping into the bowl of the separator.
The rate of flow of the culture from the bowl to the centrifuge head is adjusted according to the species of algae and the centrifugation rate of the separator. During centrifugation the algae deposit on the wall of the centrifuge head as a thick paste, and this has to be removed and resuspended in water.
Where possible, live algae are directly pumped into larval tanks. When necessary, concentrated cultures are frozen for storage; thawed algae are diluted and supplied to larval tanks through small peristaltic pumps. Living algae are considered best for feeding larvae, although frozen algae may be more convenient to handle.
Many aquaculture farms use much less elaborate methods for producing live foods, predominently algae, in outdoor or indoor tanks, using suitable fertilizers as sources of nutrients. Cultures may not always be of one species, but if the biomass produced meets the requirements of larval and/or adult feeding, it is likely to be much more economical. Large outdoor tanks of 8–40 tons capacity, with supply lines for fresh and sea water and compressed air, have been used successfully for growing selected species such as Chaetoceros. A commercial hydroponic fertilizer is added at the time of inoculation at the rate of about 20 g per cubic metre. The fertilizer is placed in bags hung in the tank. In about three to four days, an average density of 1.2 x 106 cells per ml can be expected to develop.

The ‘green water’ method of algal production is practised in some Macrobrachium culture centres (fig. 7.3). Green water is a mixed phytoplankton culture, with a predominance of Chlorella spp. Large indoor or outdoor tanksare used for its culture. The tank is fertilized once a week with a solution of a mixture of 4 parts of urea to 1 part of NPK (15 : 15 : 15) agriculture fertilizer at the rate of 185 g per 10 m3 water. In order to control the growth of filamentous algae, a small number of tilapia (at the rate of one per 400 l water) is held in the tank to graze on them. Tilapia also help to fertilize the water. Copper sulphate is applied at the rate of 0.6ppm once a week to control the growth of rotifers. Green water develops at a salinity below 12 ppt, and up to three-day-old green water can be used at the same salinity level for rearing larvae.
Related Topics