Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Heterogeneous equilibria
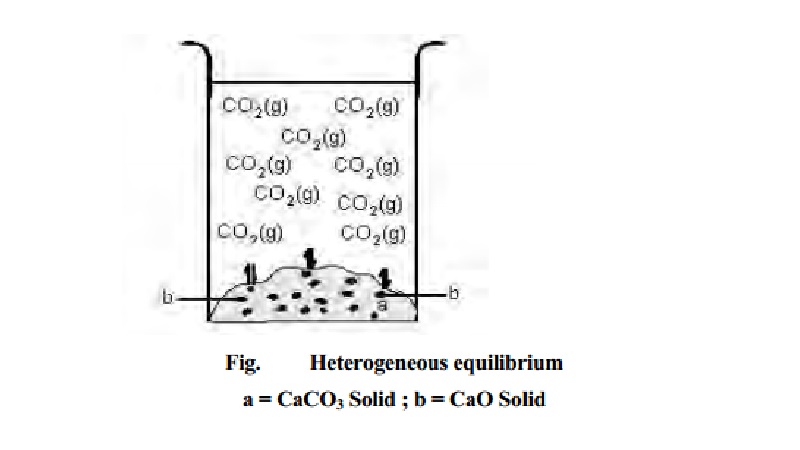
Heterogeneous
equilibria
The chemical equilibrium in which the reactants
and products are not in the same phases are called heterogeneous equilibrium. An example of heterogeneous equilibrium
can be the decomposition of calcium carbonate which upon heating forms calcium
oxide and carbondioxide under equilibrium conditions. When the reaction is
carried out in a closed vessel, the following heterogeneous equilibrium is
established.
CaCO3 -- > <
-- CaOs) + CO2(g)
The equilibrium constant expression for CaCO3 dissociation can be written as
K = [CO2] [CaO] / [CaCO3]
[CaCO3]
But CaO and CaCO3 are pure solids. The activity or
concentration of pure solids is unity.
Thus Kc = [CO2]
in terms
of partial pressures,
Kp = pco2, where Pco2 is the pressure of CO2 alone in
equilibrium. There are many examples of heterogeneous chemical equilibria with
Kp and Kc values different depending on the number of
product and reactant molecules.
(i) The
equilibrium constant expression for decomposition of liquid water would be
2H2O(l) -- > < --- 2H2(g) + O2(g)
K = [H2]2
[O2] / [H2O]2 but activity of liquid H2O = 1.0
Kc = [H2]2 [O2]
and Kp = (pH2)2
(pO2) p = partial pressure.
(ii)
Consider the equilibrium reaction of decomposition of NH4 Cl.
NH4Cl(s) -- > < -- NH3(g) + HCl(g)
Kc = [NH3] [HCl]
because [NH4Cl(5)] = 1.0
Kp = PNH3
PHCl.P=Partial Pressure
(iii)
Consider the hydrogen gas evolution equilibrium such as
3Fe(s) + 4H2O(g) -- > < -- Fe3O4 + 4H2(g)
Kc = [H2]4 / [H2O]4
kp = (pH2)2
/ (pH2O)4
since activities of Fe(G) and Fe3O4(s) are 1.0.

Related Topics