Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Group 17 - The Halogen Family
GROUP 17 - THE HALOGEN FAMILY
Group 17 of the periodic table contains the elements fluorine, chlorine, bromine, iodine and astatine. These are collectively known as HALOGENS. It is derived from two Greek words Halo and Gens meaning "Salt producer". Because most of them exist in Sea water.
General Trends
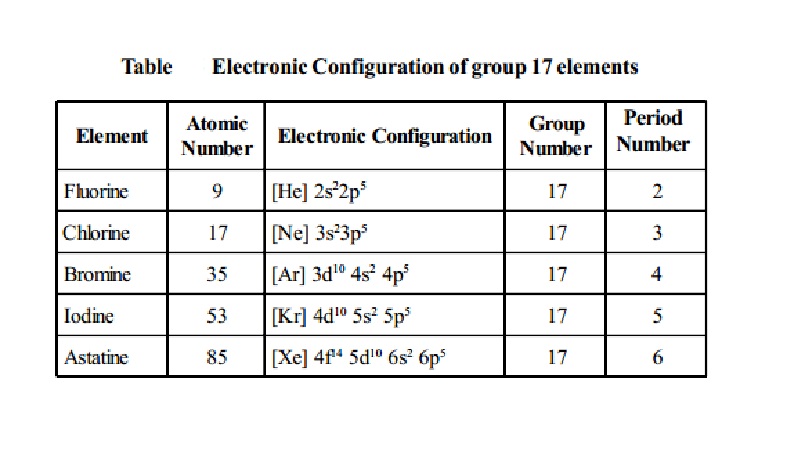
Electronic Configuration: All these elements possess ns2np5 configuration.
Table Electronic
Configuration of group 17 elements
Fluorine -
Atomic Number : 9
Electronic Configuration :
Group Number : 17 Periodic Number
: 2
Chlorine -
Atomic Number : 17
Electronic Configuration :
Group Number : 17 Periodic Number
: 3
Bromine -
Atomic Number : 35
Electronic Configuration :
Group Number : 17 Periodic Number
: 4
Iodine -
Atomic Number : 53
Electronic Configuration :
Group Number : 17 Periodic Number
: 5
Astatine -
Atomic Number : 85
Electronic Configuration :
Group Number : 17 Periodic Number
: 6
Chemical Properties
1. Oxidising power: An important feature of the halogen is their oxidising
property which is due to high electron affinity of
halogen atoms. The oxidising power
decreases from fluorine to iodine. Fluorine is the strongest oxidising agent. It oxidises other halide ions to halogens in solution or
when dry.
F2 + 2 X- ® 2F - + X2 (X- = Cl
-, Br -, I-)
Halogen of low atomic number oxidises the halide ion of
higher atomic number.
2.
Solubility: Halogens, being non-polar molecules, do
not dissolve to a
considerable extent in a polar solvent like water.
However, fluorine reacts with water readily
forming a mixture of O2 and O3.
2F2 + 2H2O ® 4HF + O2 3F2 + 3H2O ® 6HF + O3
Chlorine, bromine and Iodine are more soluble in organic
solvents such as CCl4, CHCl3 and produce yellow, brown and violet colour.
3. Hydrides of the Halogens (Hydrogen halides):
i) All halogens react with hydrogen to form volatile
covalent hydrides of formula HX.
ii) These hydrides are called hydracids.
iii) The activity of halogens towards hydrogen decreases
from fluorine to iodine. Hydrogen combines
explosively with fluorine even in dark. It combines with chlorine in the presence of sunlight and with
bromine on heating. Hydrogen combines
with iodine on heating and in presence of a catalyst.
iv) Hydracids are the reducing agents.
v) Except HF, all hydrogen halides are gases. HF is a
liquid because of inter
molecular hydrogen bonding.
H
- F ....... H -F ....... H-F ....... H-F
vi) The acidic character of HX are in the following
order.
HF < HCl < HBr < HI.
Anamalous Nature of Fluorine
Fluorine is the most reactive element among halogen. This is due to the minimum value of F-F bond dissociation energy.
Fluorine decomposes cold dilute alkalies liberating OF2 and with conc. alkali,
O2 is liberated. Under
similar conditions, the other halogens will give rise to
the hypohalites and halates respectively.
It has the greatest affinity for hydrogen, forming HF
which is associated
due to the hydrogen bonding. Hydrofluoric acid is a weak
acid whereas the other hydrohalic
acids are strong acids.
...... H- F...... H- F..... H-F.
It differs markedly from the other halogens in that it
can form two types of
salts with metals. NaF and NaHF2.
The salts of HF differ from the corresponding salts of
other hydracids. AgF
is soluble in water while the other AgX are insoluble.
Being strongly electronegative it can have only a
negative oxidation state
while the other halogens can have negative as well as
positive oxidation state.
HF attacks glass while others do not.
Fluorine, because of the absence of d-orbitals in its
valence shell does not
form any polyhalides. Thus we have I3 -, Br3-, Cl3- ions but no
F3- ion.
Etching glass
Industrially, hydrogen fluoride is obtained by heating
fluorspar (CaF2)
with
concentrated H2SO4 in a lead
vessel.
CaF2 + H2SO4® CaSO4 + 2HF.
HF distils over and the vapours are condensed in water in
a lead receiver.
Aqueous HF thus obtained is stored in wax bottles. It
cannot be stored in glass or silica
bottles as it attacks silicates and silica.
Na2 SiO3 + 6HF ® Na2SiF6 + 3H2O
SiO2 + 4HF ® SiF4 + 2H2O
The action of hydrofluoric acid on silica and silicates
is used for etching
glass. The glass article is first covered with a film on
wax. The design to be etched is now drawn
on the waxed surface and is then exposed to the action of hydrofluoric acid. Now the glass can be very soon etched.
The wax is finally washed off with
turpentine.
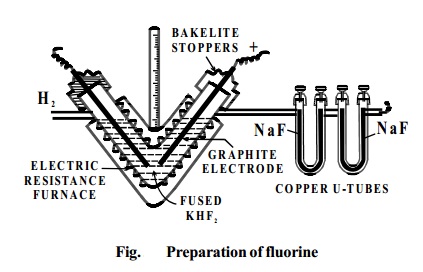
ISOLATION OF FLUORINE
Symbol - F Atomic number -9 Period Number :2
Valency -1 Atomic
mass-19 Group Number : 17
Fluorine does not occur free in nature. It occurs in the
combined form. Dennis' Method: This was devised by Dennis, Veeder and Rochow in 1931.
In this fluorine is prepared by the electrolysis of fused
sodium or potassium hydrogen fluoride
(perfectly dry) Electrolysis is carried out between graphite electrodes in a V-shaped electrically heated copper
tube. The ends of the tube are covered
with copper caps into which the graphite electrodes are fixed with bakelite cement. The copper tube is thickly lagged to
prevent loss of heat.
KHF2 ® KF + HF
HF ® H+ + F¯
2H+ + 2e- ® H
2 (At cathode)
2F
- - 2e- ® F2 (At anode)
Fluorine liberated at the anode is passed through the U-tube containing sodium fluoride. Thisremovesthe hydrogen fluoride vapourscomingwithfluorine.
NaF +HF ® NaHF2
Physical Properties
1. Fluorine is a gas and has pale greenish yellow
colour.
2. It has extremely pungent and penetrating odour. 3. It is heavier than air.
Chemical Properties
Fluorine is the most active member of halogen family.
1. Action with Hydrogen: Hydrogen explodes violently in fluorine even in
the dark.
H2 + F2 ® 2HF
2. Action with non-metals: Non-metals like carbon, silicon and phosphorus
burn in fluorine forming fluorides.
C + 2F2 ® CF4
Tetra fluoromethane
Si + 2F2 ® SiF4
Silicon tetrafluoride
2P + 5F2 ® 2PF5
Phosphorus pentafluoride
3. Action with metals: It reacts with metals forming corresponding
fluorides.
2Ag + F2 ® 2AgF
2Al + 3F2 ® 2AlF3
4. Formation of Interhalogen compounds: It forms a variety of inter halogen
compounds with other halogens.
Br2 + 3F2 ® 2Br F3
I2 + 5F2 ® 2 IF5
Uses
1. Fluorine is used in the manufacture of a series of
compounds known as
freons. These non-toxic, non-combustible and volatile
liquids are used as refrigerants in
refrigerators, deep freezers and air conditioners. The most
common, freon is known as dichlorodifluoro methane CF2 Cl2.
2. CaF2 is used
as flux in metallurgy.
3. NaF is used as a preservative to prevent fermentation
and also for preventing
dental cavities.
4. SF6 is used
as an insulating material in high voltage equipment.
5. Teflon is used as container to store hydrofluoric
acid.
6.
UF6 is used in the separation of U235 from U238.
INTERHALOGEN COMPOUNDS OR INTERHALOGENS
Each halogen combines with another halogen to form
several compounds
known as interhalogen compounds. The less
electronegative element is written first. In naming also, the less
electronegative element is mentioned first.
They are divided into four types.
AX : CIF , BrF
,BrCl, ICl, IBr.
AX3: ClF3 BrF3 ICl3
AX5 : BrF5 IF5
AX7 : IF7
They can all be prepared by direct combination or by the
action of a halogen on a lower
interhalogen, the product formed depends on the conditions.
473K
Cl2+ F2 (equal volume) ----- --- > 2ClF
(AX type)
I2 + Cl2 liquid (equi molar) ®
2ICl (AX type)
573K
Cl2 + 3F2 (excess)
---- --- > 2 ClF3 (AX3 type)
Br2 + 3F2 (diluted with nitrogen)® 2Br F3 Br2 + 5F2 (excess) ® 2Br F5 (AX5 Type)
I2 + 5F2 (Excess) ® 2IF5 (AX5 Type)
IF5 + F2 (Excess)
------- 573K ----- --- > IF7 (AX7 Type)
The bonds are essentially covalent because of the small
electronegativity
difference, and the melting and boiling points increase
as the difference in electronegativity
increases.
The interhalogens are generally more reactive than the
halogens (except F)
because the A-X bond is weaker than the X-X bond in the
halogens. The reactions are similar to
those of the halogens. Hydrolysis gives halide and oxyhalide ions, the
oxyhalide ion being formed from the larger halogen present.
BrF5 + 3 OH- ---
--- > 5F¯ + BrO3¯ + 3 H+
Bromate
ICl + OH- --- - ----------- --- > Cl ¯ + OI ¯ + H+
hypoiodite
Structures of interhalogen compounds
Interhalogen compounds are generally covalent compounds in which the larger halogen forms the central atom.
1. Type AX. As excepted, the compounds of the type AX are linear. Thus CIF, BrF, BrCl, IBr and ICI are all linear in structure.
Electronic structure of Chlorine atom, in the ground state and hybridised state is represented as in Fig.
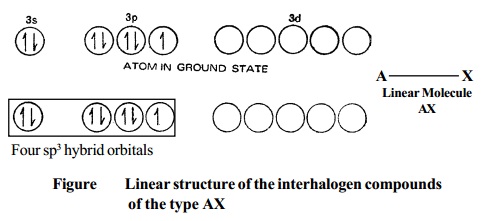
Although the spatial arrangement of the four electron pairs (bp = 1 and lps = 3) round the central chlorine atom is tetrahedral, due to the presence of three lone pairs of electrons in three hybrid orbitals, the shape of AX molecule gets distorted and become linear.
2. Type AX3 Compounds of the type AX3 have trigonal bipyramidal structure,
Fig. for the ClF3 molecule.
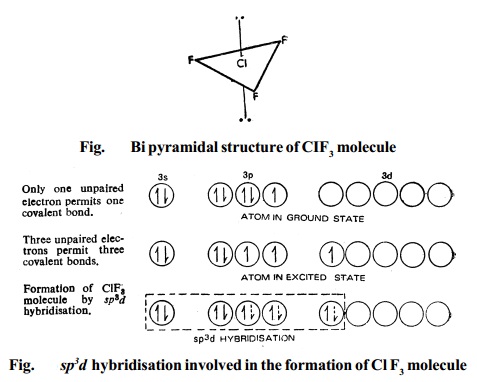
Bipyramidal structure arises out of sp3d hybridisation involved in the formation of this compound, as illustrated in the Fig.. The three dotted arrows indicate electrons contributed by the three fluorine atoms (without lone pair it is T-shaped).
3. Type AX5 (IF5, BrF5, etc.) These
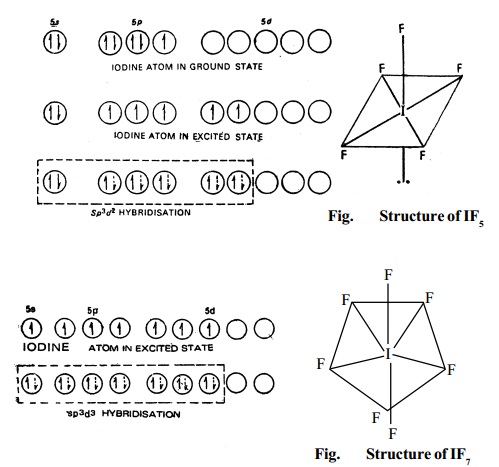
compounds are formed by sp3d2 hybridisation and hence have an octahedral
structure, as shown in Fig. for the formation of IF5 molecule (without lone pair it is square
pyramidal).
4. Type AX7 (IF7). This compound has
a pentagonal bipyramidal structure since
this is formed by sp3d3hybridisation.
Problem
An element A occupies group number 17 and period
number 2, shows
anomalous behaviour. A reacts with water forms a
mixture of B, C and acid D. B and C are allotropes.
A also reacts with hydrogen violently even in dark togive an acid D. Identify A,B,C and D.
write the reactions.
Solution
i)
The element A that occupies group number 17 and period number 2 is
fluorine.
ii) Fluorine reacts with water and forms a mixture of
B and C
2F2 + 2H2O ® 4HF + O2
3F2 + 3H2O ® 6HF + O3
Therefore, B is Oxygen and C is Ozone.
iii) Fluorine reacts with hydrogen to give D.
F2 + H2 ® 2HF
D is
Hydrofluoric acid.
Related Topics