Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Dissociation of PCl5 - Equilibrium constants in terms of degree of dissociation
Degree of dissociation
(x)
In the
study of dissociation equilibrium, it is easier to derive the equilibrium
constant expression in terms of degree
of dissociation (x). It is considered as the fraction of total molecules
that actually, dissociate into the simpler molecules x has no units. If x is
the degree of dissociation then for completely dissociating molecules x = 1.0.
For all dissociations involving equilibrium state, x is a fractional value. If
x is known, Kc or Kp can be calculated and vice-versa.
Equilibrium constants in terms of degree of
dissociation
Dissociation of PCl5
Phosphorus
pentachloride dissociates in gas phase to give PCl3 and Cl2.
This is an example of gaseous homogeneous equilibrium. It can be represented as
PCl5(g) -- > <
-- PCl3(g) + Cl2(g)
For this
reaction ∆n=1 Kp=Kc(RT)
Let us consider that one mole of PCl5 is present initially in a vessel
of volume V dm3. At equilibrium let x mole dissociates to give x
mole of PCl3 and x mole of Cl2. The equilibrium
concentrations of the components can be given as follows :
PCl5(g) PCl3(g) Cl2(g)
Initial
number of moles 1 0 0
Number of
moles reacted x - -
Number of
moles at equilibrium 1-x x
x
Equilibrium
concentration 1-x/V x/V x/V
According
to law of mass action,
Kc = [PCl3][Cl2] / [PCl5]
Substituting the
values of equilibrium
concentrations in the
above equation we get, Kc = (x/V)(x/V) / (1-x)/V
= x2V/V2(1-x)
= x2/
(1-x)V
Derivation of Kp in terms of x
Let us
consider that one mole of PCl5 is present initially. At equilibrium, let us
assume that x mole of PCl5 dissociates to give x mole of PCl3 and x
mole of Cl2. Let the total pressure at equilibrium be P atmosphere.
The number of moles of PCl5, PCl3 and Cl2
present at equilibrium can be given as follows :
PCl5(g) PCl3(g) Cl2(g)
Initial
number of moles 1 0 0
Number of
moles reacted x - -
Number of
moles at equilibrium 1-x X x
Total number of moles at equilibrium = 1 -
x + x + x
= 1
+ x
We know
that partial pressure is the product of mole fraction and the total pressure.
Mole fraction is the number of moles of that component divided by the total
number of moles in the mixture. Therefore
PPCl4
=(1-x)P / 1+x
PPCl3
=(x)P / 1+x
PPCl2
=(x)P / 1+x
We know
that Kp = PPCL3.PCL2/PPCL5
substituting
the values of partial pressures in this expression
Kp
= x2.P2/(1+x)2
. (1+x)/(1+x) . 1/P
Kp
= x2P / 1-x2
When x
< < 1, x2 value can be neglected when compared to one.
\ Kp ~ x2P
This equation can be used to predict the influence
of pressure on this equilibrium.
Influence of pressure
: The expression for Kc contains the volume term
and the expression for Kp contains the pressure term. Therefore this
equilibrium is affected by the total pressure. According to the above equation,
increase in the value of P will tend to increase the value of Kp. But Kp is a
constant at constant temperature. Therefore, in order to maintain the constancy
of Kp the value of x should decrease. Thus, increase in total
pressure favours the reverse reaction and decreases the value of x.
Influence of
concentration : Increase in the concentration of PCl5 favours the forward reaction, while
increase in the concentration of either PCl3 or Cl2
favours the reverse reaction. Increase in the concentration of a substance in a
reversible reaction will favour the reaction in that direction in which the
substance is used up.
Influence of catalyst
: A catalyst will affect the rates of the forward and reverse reactions to the
same extent. It does not change either the amount of reactants and products
present at equilibrium or the numerical value of Kp or Kp. However, the
equilibrium is obtained quickly in the presence of a catalyst.
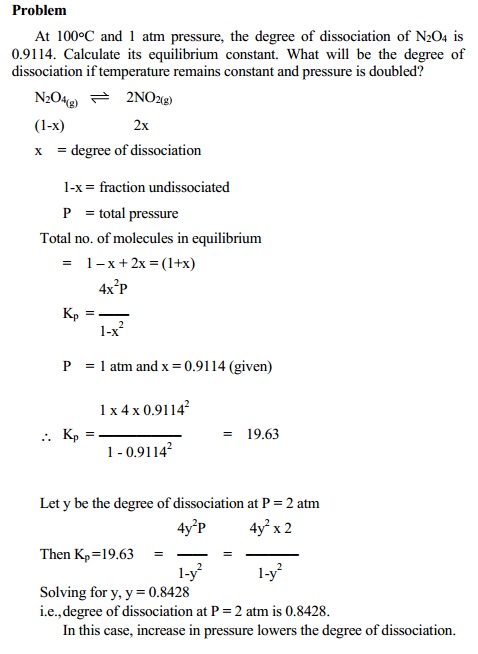
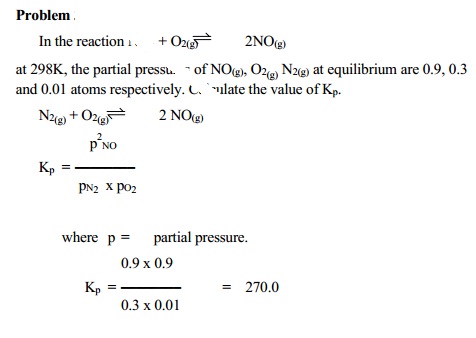
Related Topics