Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Determination and Calculation of lattice enthalpy of NaCl
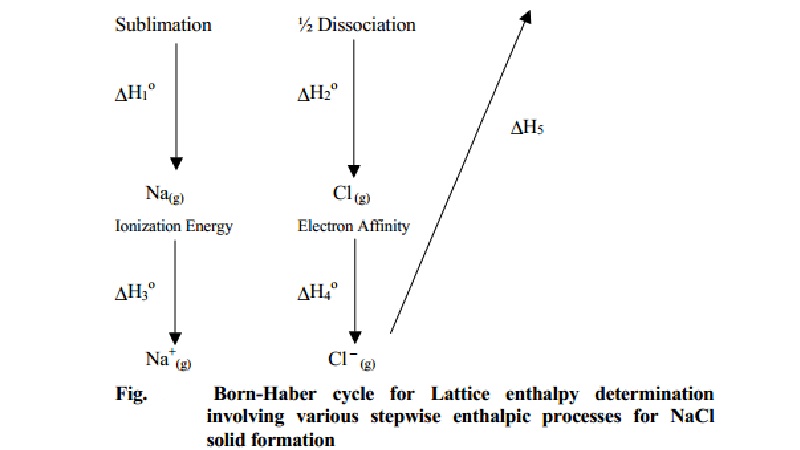
Born Haber's Cycle
Determination of
Lattice enthalpy
It is not possible to calculate the lattice
enthalpy directly from the forces of attraction and repulsion between ions but
factors associated with crystal geometry must also be included. The solid
crystal is a three-dimensional entity. The lattice enthalpy is indirectly
determined by the use of Born - Haber Cycle. The procedure is based on Hess's
law, which states that the enthalpy change of a reaction is the same at
constant volume and pressure whether it takes place in a single or multiple
steps long as the initial reactants and the final products remain the same. Also
it is assumed that the formation of an ionic compound may occur either by
direct combination of elements (or) by a step wise process involving
vaporisation of elements, conversion of gaseous atoms into ions and the
combination of the gaseous ions to form the ionic solid.
For example consider the formation of a simple ionic solid such as an
alkali metal halide MX, the following steps are considered.
M(s)--- (∆ H0(1) )--- > M(g)
--- (∆ H0(3) )--- > M+(g) + e
M(s) + ½ X2(g) --- (∆ H0(2)
)- > X(g) -- (∆ H0(4) , +e ) -- > X-(g)+
M+(g) ---- > MX(s) --- (∆ H0(f)
) -- > ½ X2(g)
∆ H0(1) = enthalpy
change for sublimation of M(s) to M(g)
∆ H0(2) = enthalpy
change for dissociation of 1/2 X2(g) to X(g)
∆ H0(3) = ionization
energy of M(g) to M+(g)
∆ H0(4) = electronic
affinity or electron gain energy for conversion of X(g) to X-(g)
∆ H0(5) = the
lattice enthalpy for formation of solid MX (1 mole).
∆ H0(f) = enthalpy
change for formation of MX solid directly from the respective elements such as
1 mole of solid M and 0.5 moles of X2(g).
∆ H0(f) = ∆
H0(1) + ∆ H0(2) +
∆ H0(3) + ∆ H0(4)
+ ∆ H0(5)
Some important
features of lattice enthalpy are:
The greater the lattice enthalpy the more stabler the ionic bond formed.
The lattice enthalpy is greater for ions of higher charge and smaller
radii.
The lattice enthalpies affect the solubilities of ionic compounds.
Calculation of lattice
enthalpy of NaCl
Let us use the Born - Haber cycle for
determining the lattice enthalpy of NaCl as follows :
The standard enthalpy change, ∆ H0(f)
overall for the reaction,
Na(s) + 1/2 Cl2(g) → NaCl(s) is - 411.3 kJmol-1
Na(s) + ½ Cl 2(g) --- (∆f Ho ) -- > NaCl(s)
Since the reaction is carried out with reactants in elemental forms and
products in their standard states, at 1 bar, the overall enthalpy change of the
reaction is also the enthalpy of formation for NaCl. Also, the formation of
NaCl can be considered in 5 steps.The sum of the enthalpy changes of these
steps is considered equal to the enthalpy change for the overall reaction from
which the lattice enthalpy of NaCl is calculated.
Atomisation :
∆ H0(1) for Na(s)
→ Na(g) is + 108.70 (kJ mol1)
Dissociation:
∆ H0(2) for ½ Cl2(g) -- -- > Cl(g) is + 122.0
Ionisation :
∆ H0(3) for
Na(g) -- --
> Na+(g)
+ e is + 495.0
Electron affinity :
∆ H0(4) for
e + Cl(g) -- -- >
Cl- (g) is - 349.0
Lattice enthalpy :
∆ H0(1) for Na+(g)
+ Cl-(g) -- -- > NaCl(g) is ?
∆ H0(f) = ∆
H0(1) + ∆ H0(2) +
∆ H0(3) + ∆ H0(4)
+ ∆ H0(5)
∆ H0(5) = -788.0 kj mol-1
But the lattice enthalpy of NaCl is defined by the reaction
NaCl(g) → Na+ (g) + Cl- (g) only.
Lattice enthalpy value from ∆ H0(5) is written
with a reversed sign.
∴
Lattice enthalpy of NaCl = +788.0 kJ mol-1.
Related Topics