Chapter: Biology of Disease: Disorders of the Immune System
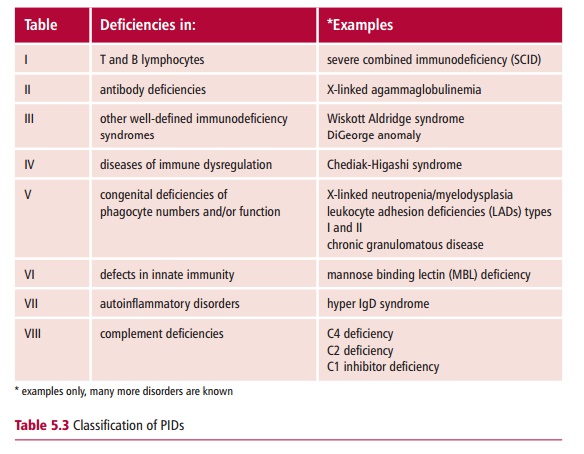
Complement deficiencies - Primary Immunodeficiency Disease
Complement deficiencies
The role of complement in immune defense was
outlined. Here it will be described in more detail. The activation of
complement results in the:
·
lysis of bacteria;
·
stimulation of the inflammatory response;
·
promotion of phagocytosis;
·
clearance of immune complexes.
The value of these roles cannot be overestimated.
Complement may be activated by one of three pathways: the classical pathway,
which is activated by IgG or IgM, following binding to an antigen; the
alternative pathway, which is stimulated principally by components of the cell
walls of bacteria and yeasts; and the lectin pathway, which is initiated by the
binding of mannose binding lectin (MBL) to bacteria. Complement proteins C1, 2,
3 and 4 are involved in classical activation, while Factors B, D, H, I and P
are involved in the alternative pathway activation. Activation of the lectin
pathway requires MBL, MBL-associated serine proteases (MASP) 1 and 2 and C2, C3
and C4. All three pathways can lead to the production of membrane attack
complexes (MACs), which are composed of C5b, C6, C7, C8 and C9. These cause
lysis of the pathogen; in addition, fragments of activated complement proteins,
such as C3a, C4a, C5a, promote inflammation by binding to mast cells and
causing them to degranulate and release inflammatory chemicals, such as
histamine . These chemicals may also promote the chemotaxis of neutrophils out
of the inflamed vessels. Other complement proteins, such as C1q, C3b and C567
act as opsonins. The absence of any of these proteins can severely compromise
health. Complement is potentially very inflammatory once activated and there
are a number of regulatory molecules which either prevent unregulated activation
of complement, such as C1 inhibitor (C1INH), or which prevent damage to
innocent bystander cells, such as Decay Accelerating Factor (DAF).
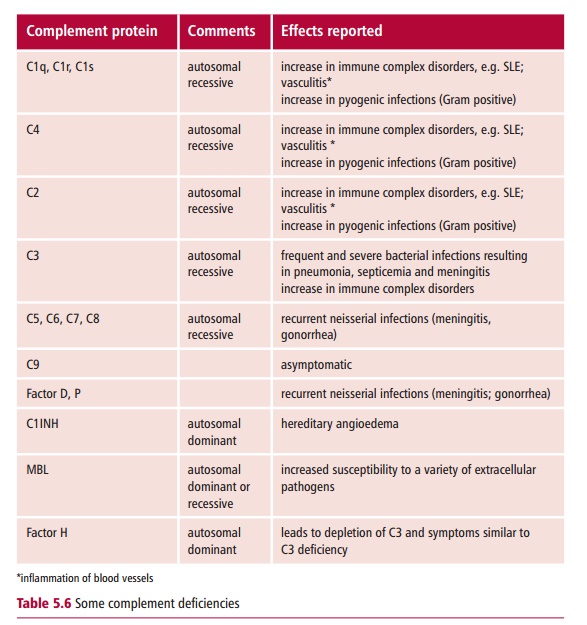
Inherited deficiencies of each complement protein
have been described (Table5.6).
Deficiencies in several of the early classical pathway proteins result inan
increased incidence of immune complex disorders, showing the role of complement
in helping to remove immune complexes from the circulation. Over 90% of
patients with a homozygous C1q deficiency and 10% of patients with a homozygous
C2 deficiency develop SLE. A deficiency of MBL is associated with increased
incidence of infections with, for example, Pseudomonas
aeruginosa. Newborn babies and infants are particularly at riskfrom this
deficiency, indicating the importance of this pathway in protecting the young
from infection.
Deficiencies in the classical and the alternative
pathways can be detected in the laboratory by carrying out the CH50 and AP50 tests
respectively. The CH50 test measures the ability of the
patient’s serum to lyse antibody-coated sheep erythrocytes, while the AP50 determines its
ability to lyse these uncoated cells in rabbits. Results are expressed in terms
of the ability of the serum to induce lysis of 50% of the target erythrocytes.
Individual complement proteins can be measured by immunoassay, using specific
antibodies. Thus C3 and C4 can be measured by nephelometry, single radial
immunodiffusion or by ELISA .
Hereditary angioedema (HAE) results from faults in
the activity of the complement regulator, C1 inhibitor (C1INH). This protein
normally functions to prevent the overactivation of the first part of the
classical pathway by inhibiting C1r and C1s. The lack of the inhibitor leads to
consumption of C4 and C2. Two types of HAE occur. Type I results from reduced
levels of C1INH while in Type II the inhibitor is present but nonfunctional.
The condition is an autosomal dominant disorder, which has an incidence of one
in 50 000 to 150 000, with 85% of cases being Type I.
The disorder presents as noninflammatory and painless
swellings of the skin, especially that of the limbs, which is often
precipitated by physical trauma and anxiety. Abdominal pain is caused by the
involvement of internal organs, such as the stomach, bladder and intestines.
Severe edema of the larynx can cause death. Treatment during attacks is to
administer fresh frozen plasma or commercially available C1-INH. Prophylactic
treatment with danazol, an androgen, has been shown to stimulate the synthesis
of C1-INH, but prolonged treatment may result in unpleasant side effects, such
as virilization in women and suppression of testosterone production in men .
Related Topics