Chapter: Modern Analytical Chemistry: Obtaining and Preparing Samples for Analysis
Classifying Separation Techniques: Separations Based on a Change of State
Separations Based on a Change of State
Since an analyte and interferent are usually in the same phase, a separation often can
be effected by inducing a change in one of their physical
or chemical states. Changes in physical state
that have been exploited for the purpose
of a separation include liquid-to-gas and solid-to-gas phase
transitions. Changes in chemical state involve one or more chemical reactions.
Changes in Physical State
When the analyte
and interferent are mis-
cible liquids, a separation based
on distillation may
be possible if their
boiling points are
significantly different. The
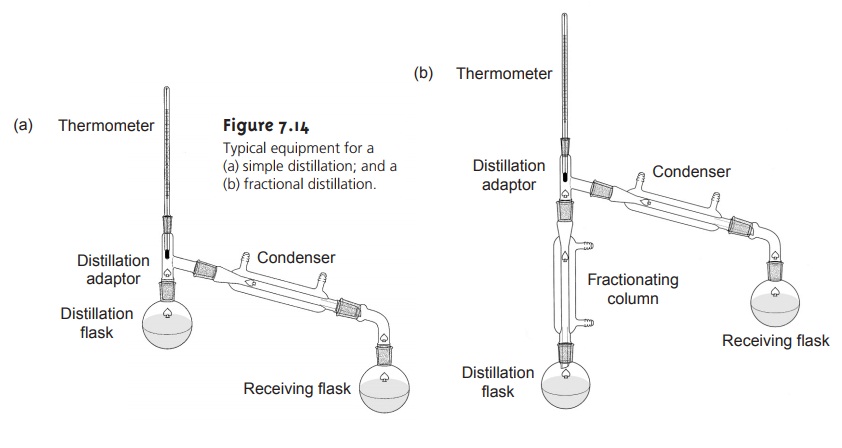
progress of a distillation is outlined in Figure
7.13, which shows
a plot of temperature versus
the vapor-phase and liquid-phase composition of a mixture consisting of a low-boiling analyte and a high-boiling interferent. The initial
mixture is indicated by the point labeled A. When this solution is brought to its boiling
point, a vapor
phase with the
composition indi-cated by the point labeled B is in equilibrium with the original
liquid phase. This
equilibrium is indicated by the horizontal tie-line between
points A and B. When
the vapor phase
at point B condenses, a new liquid phase with the same composition
as the vapor phase (point C) results. The
liquid phase at point C boils at a lower
temperature, with an equilib-
rium established with the vapor-phase composition indicated by point D. This
process of repeated vaporization and condensation gradually separates the ana-
lyte and interferent.
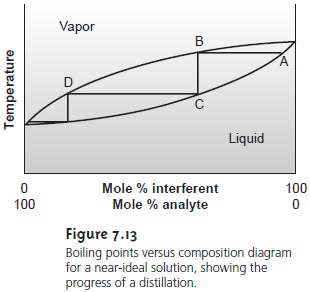
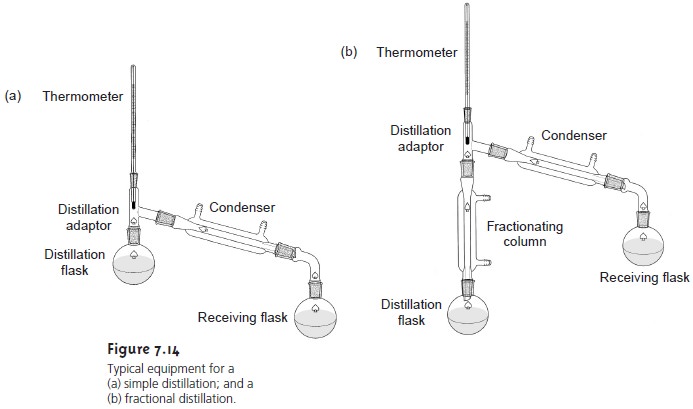
Two examples of the equipment
used for distillations are shown in Figure 7.14. The
simple distillation apparatus shown in Figure
7.14a does not produce a very effi- cient separation and is useful only for separating a volatile liquid from nonvolatile liquids or for separating liquids with boiling
points that differ
by more than 150 °C. A
more efficient separation is achieved by a fractional distillation (Figure 7.14b). Packing the distillation column
with a high-surface-area material, such as a steel
sponge or glass beads, provides more opportunity for
the repeated process
of vapor- ization and
condensation necessary to effect a complete separation.
When the sample is a solid, a separation of the analyte and interferent by subli- mation may be possible. The sample is heated at a temperature and pressure below its triple point where the solid vaporizes without passing through the liquid state. The vapor is then condensed to recover the purified solid. A good example of the use of sublimation is in the isolation of amino acids from fossil mollusk shells and deep-sea sediments.
Another approach for purifying solids
is recrystallization. The solid is dissolved
in a minimum volume of solvent, for
which the analyte’s solubility is significant when the solvent is hot, and minimal when the solvent
is cold. The interferents
must be less soluble in the hot solvent than the analyte,
or present in much smaller amounts. A portion
of the solvent is heated in an Erlenmeyer
flask, and small amounts of sample are added until
undissolved sample is visible. Additional heated solvent is added until the sample is again dissolved
or until only insoluble impuri- ties remain. The process
of adding sample
and solvent is repeated until
the entire sample has been added to the Erlenmeyer flask. If necessary, insoluble impurities
are removed by filtering the heated solution. The solution is allowed to cool slowly, promoting the growth of large, pure crystals, and then cooled in an ice bath to min- imize solubility losses. The purified sample is isolated
by filtration and rinsed to re-
move soluble impurities. Finally, the sample is dried to remove any remaining
traces of the solvent. Further
purification, if necessary, can be accomplished by ad- ditional recrystallizations.
Changes in Chemical State
Distillation, sublimation, and recrystallization use a
change in physical state as a means
of separation. Chemical
reactivity also can be
used in a separation by effecting a change in the chemical
state of the analyte or in-
terferent. For example, SiO2 can be separated from a sample
by reacting with
HF. The volatile SiF4 that forms is easily removed by evaporation. In other cases
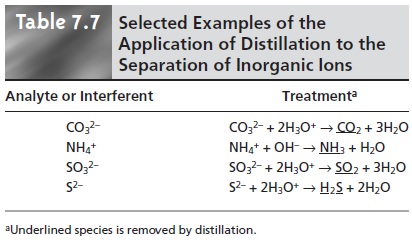
distilla- tion may be used to remove a nonvolatile inorganic
ion after chemically converting it to a more volatile form. For example,
NH4+ can be separated from a sample by mak ing the
solution basic, resulting in the formation of NH3.
The ammonia that is produced can then be removed by distillation. Other examples are
listed in Table 7.7.
Other types of reactions can be used to chemically separate an analyte and
interferent, including precipita- tion, electrodeposition, and ion exchange. Two impor- tant examples of the application of precipitation are the pH-dependent solubility of metal oxides
and hydroxides, and the solubility of metal sulfides.
Separations based on the pH-dependent solubility of oxides
and hydroxides are usually accomplished using strong
acids, strong bases,
or NH3/NH4Cl buffers.
Most metal oxides and hydroxides are soluble in hot concentrated HNO3, although a few
oxides, such as WO3, SiO2, and SnO2 remain
insoluble even under
these harsh con- ditions. In determining the
amount of Cu in brass,
for example, an interference
from Sn is avoided by dissolving the sample with a strong
acid. An insoluble residue of SnO2 remains
that can then be removed
by filtration.
Most metals will precipitate as the hydroxide in the presence of concentrated NaOH. Metals forming amphoteric hydroxides, however, remain soluble in concen-
trated NaOH due to the formation of higher-order hydroxo-complexes. For exam- ple, Zn2+ and Al3+ will not
precipitate in concentrated NaOH due to the formation of
Zn(OH) – and Al(OH) –. The solubility of Al3+ in
concentrated NaOH is used to isolate aluminum from
impure bauxite, an ore of Al2O3. The
ore is powdered and placed in a solution of concentrated NaOH where the Al2O3 dissolves to form Al(OH)
–. Other oxides that may be present in the ore, such as Fe O and SiO
, re- main insoluble. After filtering, the filtrate is acidified to recover the aluminum as a
precipitate of Al(OH)3.
The pH of an NH3/NH4Cl buffer
(pKa = 9.24) is sufficient to ensure the
precip- itation of most
metals as the
hydroxide. The alkaline earths and alkaline metals, however, will not precipitate at this pH. In addition, metal ions that form soluble complexes with NH3, such as Cu2+,
Zn2+, Ni2+,
and Co2+, also will not precipitate
under these conditions.
Historically, the use of S2– as a precipitating reagent
is one of the earliest
exam- ples of a separation technique. In Fresenius’s 1881 text, A System of Instruction in Quantitative Chemical Analysis,15 sulfide is frequently used as a means for separat-
ing metal ions from the
remainder of the
sample matrix. The
importance of sulfide as a precipitating reagent
for separations is due to two factors:
most metal ions,
ex- cept for the alkaline earths
and alkaline metals,
form insoluble sulfides; and the sol- ubilities of these metal
sulfides show a substantial variation. Since the concentration of S2– is pH-dependent, control of pH was used to determine which
metal ions would precipitate. For example, in Fresenius’s gravimetric procedure for the deter-
mination of Ni in ore samples (see Figure 1.1 for a schematic diagram of this procedure), sulfide
is used three
times as a means of separating Co2+ and
Ni2+ from Cu2+ and, to a lesser
extent from Pb2+.
Related Topics