Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Classification and Electronic configuration of d-block Elements
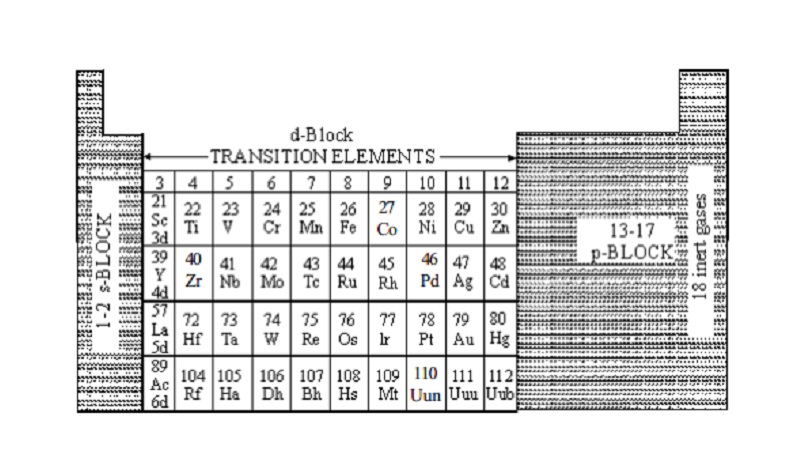
The d-block elements are located in the middle of the
periodic table and consists of metals
only. It consists of four series, each series consists of 10 elements.
In these elements, the last electron enters the d
orbital of the penultimate shell i.e.
the last electron goes to (n-1) d orbital. Hence these elements are named as d-block elements. These elements have partly filled
d-subshells in their elementary form or
in their simple ions. The d-block elements are called transition elements
because these represent a transition from highly electropositive elements (metals) of s-block to least electropositive elements
(non-metals) of p-block.
Classification of d-block Elements
Based on whether the last electron goes to 3d,4d,5d or 6d
orbital, d-block
elements are classified into four series. They are
i) 3d
series or First transition series (21Sc to 30Zn)
ii) 4d series or Second transition series (39Y to 48Cd)
iii) 5d series or Third transition series (57La and 72Hf to 80Hg)
iv) 6d series or Fourth transition series (89Ac and 104Rf to 112)
or Incomplete series.
Electronic configuration of d-block Elements
In the transition elements, d-orbitals of penultimate
shell are successively filled. The first transition series involves the filling
of 3d orbitals. It starts from scandium (Z=21) and goes up to zinc (Z=30).
The second transition series involves the filling of
4d-orbitals and includes 10 elements from
yttrium (Z=39) to cadmium (Z=48).
The third transition series involves filling of
5d-orbitals. The first element of this series is lanthanum (Z=57). It is followed by
fourteen elements called lanthanides which
involve the filling of 4f-orbitals. The next nine elements from hafnium (Z=72) to mercury (Z=80) belong to third
transition series.
The general electronic configuration of transition
elements is (n-1)d1-10 ns1-2.
Related Topics