Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Chromium: Occurrence, principles of extraction, Properties, Uses and alloys
Occurrence and principles of extraction of chromium Chromium
Atomic mass : 51.99 Valency
: 0,1,2,3,4,5,6
Atomic number : 24 Symbol : Cr
Position in the periodic table : Period Number -4, Group
Number -6.
L.N. Vanquelin, a french chemist discovered a new element
in 1797, while examining a mineral
found in Siberia. It was named chromium because it forms coloured compounds [Greek word - chroma - colour]
Occurrence
Metallic chromium does not occur in the native state. In
India chromite ore occurs in Bihar, Mysore, Chennai and Bombay.
Ores
The important ore of chromium is Chromite or chrome ore, FeO Cr2O3
The chief ore of chromium is chromite ore.
Extraction of chromium metal from chromite ore
The extraction of chromium metal from chromite ore
consists of the following steps.
1. Concentration
The crushed ore is concentrated by gravity separation
method.
2. Conversion of the concentrated chromite ore into
Na2CrO4
The concentrated ore is mixed with excess of Na2CO3 and a small amount of lime and roasted in a reverberatory furnace at
900-1000 o C in the presence of free
supply of air. During this process, chromite ore is converted into soluble sodium chromate.
4(FeO. Cr2O3) + 8Na2CO3 + 7O2 (from
air)
Chromite ore ¯ 900-1000 o C
8Na2CrO4 +2Fe2O3 + 8CO2
Soluble Insoluble
Conversion of Na2CrO4 into Na2Cr2O7
The solution containing Na2CrO4 is treated with a calculated quantity of
H2SO4,
Na2CrO4 is converted into Na2Cr2O7.
2Na2CrO4 + H2SO4 ® Na2Cr2O7 + Na2SO4 + H2O
Conversion of Na2Cr2O7 into Cr2O3
Na2Cr2O7 is heated with carbon to get sodium chromite,
Na2Cr2O4 which
on treatment with H2O, gives Cr2O3 precipitate.
Na2Cr2O7 + 3C ® Na2Cr2O4 + 3CO
Na2Cr2O4 + H2O ® Cr2O3¯ + 2 NaOH
Reduction of Cr2O3 to
chromium metal
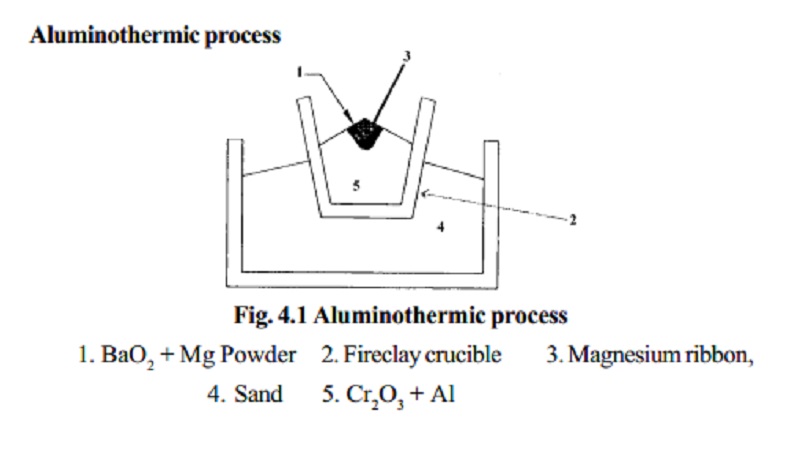
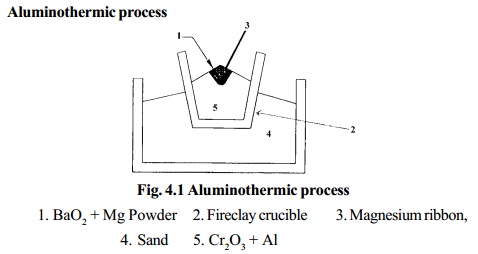
Aluminothermic process
1. BaO2 + Mg
Powder
2. Fireclay crucible
3. Magnesium ribbon,
4. Sand
5. Cr2O3 + Al
Chromic oxide is mixed with powdered Aluminium in the
ratio 3:1 and is
placed in a large fire clay crucible. A mixture of barium
peroxide and Mg powder is placed over
this. The crucible is surrounded by sand which prevents loss of heat by
radiation. The mixture is ignited by a piece of Mg ribbon. During this
process a large amount of heat is liberated, in which Cr2O3 is reduced to chromium.
The molten chromium is collected in the crucible and
aluminium oxide is removed
as slag.
Cr2O3 + 2Al ®2Cr + Al2O3 + 468.6
kJ
Properties of Cr
Physical Properties
1. The metal is silvery white and crystalline. 2. It is very hard and brittle 3. It melts at 2113K.
Chemical Properties
1. Action of air : It is unaffected by air at ordinary temperatures.
When heated to very high temperature at about 2000 o C it
is oxidised to
chromic oxide.
4Cr
+ 3O2 ® 2Cr2O3
2. Action of Water : There is no action at
ordinary temperatures. However it
decomposes steam at red heat to give chromic oxide and
hydrogen.
2Cr
+ 3H2O ® Cr2O3 + 3H2
3. Action of Acids : It dissolves in dilute
hydrochloric acid and sulphuric acid to
liberate hydrogen and forms chromous salts.
Cr + 2HCl ® CrCl2 + H2
Cr + H2SO4 ® CrSO4 + H2
4. With hot concentrated sulphuric acid it gives chromic
sulphate and liberates sulphur dioxide.
2Cr + 6H2SO4 ® Cr2(SO4)3 + 3SO2 + 6H2O
5. Dilute nitric acid does not attack the pure metal
while concentrated acid renders it inactive
or passive i.e., it does not show its usual reactions.
6. Action with Halogens : Chromium combines
directly with fluorine and dry chlorine to
give chromium (III) halides.
2Cr + 3F2 ® 2CrF3
2Cr + 3Cl2 ® 2CrCl3
Use : In chrome -
plating.
1. The articles to be plated with chromium are made the
cathode in an electrolytic bath
consisting of chromic acid and sulphuric acid while the anode is made of a plate of lead. During electrolysis chromium deposits
on the article (cathode).
Generally the articles are first plated with nickel and then subjected to chromium plating.
2. In the manufacture of alloy steels (e.g.) chrome steel,
chrome vanadium steel, stainless
steel and tungsten steel.
3. Chrome nickel steel is used for armour plates.
4. Chromium salts are used as mordants and in the
manufacture of coloured glass and pottery.
5. Chromium compounds are used in dyeing as pigments and in
tanning of leather.
Alloys of chromium
Alloy %
composition Uses
i) Ferrochrome -
Cr = 65% Fe = 35%
It is used in manufacture of chrome steel, burglar proof safe
ii) Stainless steel
Cr = 11-13%
C = 0.1 - 0.4%
Fe = 73% Ni = 8%
It is used for cutlery and house hold wares.
iii) Nichrome
Cr = 15%, Ni = 60%
Fe = 25%
It is used in resistance wires for electrical heating
iv) Stellite
Cr = 20-35%
Co = 40-80%
Ni = 0.25%
C = 0.75 - 2.5%
It is used in cutlery, surgical instruments,.....etc.
Related Topics