Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Canal rays and Properties of Positive rays (or) Canal rays
Positive rays (or) Canal rays
While conducting experiments on the gas
discharge, in 1886, German Physicist, E.Goldstein, discovered that
if the cathode used is perforated, luminous streams appe ared in the tube behind the cathode. These streams were
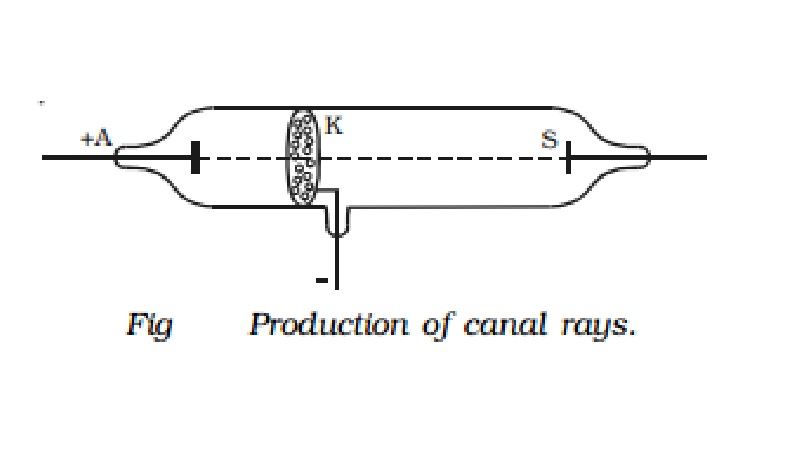
called as canal rays. The discharge tube designed by Goldstein is shown in Fig.
The tube contains an anode (A), a perforated cathode (K) and a fluorescent
screen (S). At a pressure of about 1mm of mercury, a luminous stream of
particles were observed behind the cathode proceeding in a direction opposite
to that of the cathode rays. Goldstein, called them as canal rays, since they
pass through and emerge from the holes, in the cathode in straight lines,
opposite to the direction of the cathode rays. From the nature of the
deflection produced, by a magnetic field or electric field, these rays were
found to be positively charged particles. Hence, canal rays are most commonly
known as positive rays.
Properties
of Canal rays
i.
They are the streams of positive
ions of the gas enclosed in the discharge tube. The mass of each ion is nearly
equal to the mass of the atom.
ii.
They are deflected by electric and
magnetic fields. Their deflection is opposite to that of cathode rays.
iii.
They travel in straight lines.
iv.
The velocity of canal rays is much
smaller than the velocity of cathode rays.
v.
They affect photographic plates.
vi.
These rays can produce fluorescence.
vii.
They ionize the gas through which
they pass.
Related Topics