Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Attainment Of Equilibrium In Chemical Reactions
ATTAINMENT OF EQUILIBRIUM IN CHEMICAL REACTIONS
A chemical reaction in equilibrium involves the opposing
reactions. One of the reactions produces the products and is known as the
forward reaction while the other produces
the reactants from products and is known as the reverse reaction. Chemical equilibrium is dynamic in nature. At
equilibrium, reactant and product
molecules are both present in the reaction mixture in definite amounts. The equilibrium concentrations of the reactants and
products do not change under constant
temperature, pressure and catalysts etc.
Consider a general equilibrium
reaction at constant temperature represented By
aA + bB -- kf -- > < -- kr -- cC + dD
According to law of mass action, the rate of forward
reaction is
Rf = kf [A]a [B]b
and the rate of reverse reaction is
Rr = kr [C]c [D]d
where kf and kr are the rate constants of the forward and
reverse reactions respectively.
At equilibrium Rf= Rr
∴ kf [A]a [B]b = kr [C]c [D]d
k f / k r = [C]c [D]d / [A]a [B]b
kf / k r = Kc = equilibrium constant
Kc =
[C]c [D]d / [A]a [B]b
This equation of Kc is also known as equilibrium law equation. Kc is the equilibrium
constant expressed in terms of molar concentrations of reactants and products. For reactions involving gaseous reactants or
products or both, it is
more convenient to use partial pressures instead of molar
concentrations. Thus Kc in equilibrium law equation becomes Kp which is the equilibrium constant expressed in terms of partial pressure.
1. Relationship between Kp and Kc
Consider a general chemical equilibrium reaction in
which the reactants and products are in
gaseous phases,
aA + bB + cC + ........ lL + mM +
nN + .....
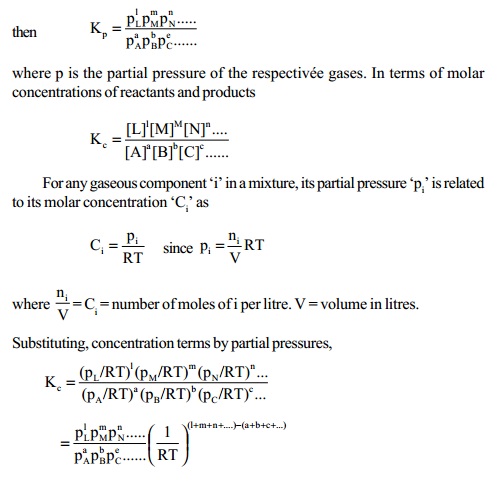
Kp = Kc(RT) Dng
where Dng = total number of
stoichiometric moles of gaseous products - total number of stoichiometric moles of gaseous reactants.
Usually, depending on the sign of Dng,
Kp and Kc are related in three ways.
Case (i)
When Dng = 0, the total number of
moles of gaseous products are equal to the
total number of moles of gaseous reactants.
For example, in the formation equilibrium of HI,
H2(g) + I2(g) -- kf -- > < -- kr -- 2HI(g)
Dng = 2-(1+1) = 2-2 =0
∴ Kp = Kc (RT)O
Kp = Kc
Case (ii)
When Dng = +ve, the total number
of moles of gaseous products are greater than
the total number of moles of gaseous reactants.
For example,
2H2O(g) + 2Cl2(g) ) -- kf -- > < -- kr -- 4HCl(g) + O2(g)
Dng = (4+1) - (2+2)
= 5-4=1
∴
Kp = Kc (RT)1
Kp = Kc RT
and Kp> Kc
Case (iii)
When Dng = -ve, the total number
of moles of gaseous products are lesser than the total number of moles of gaseous reactants.
For example, consider the formation equilibrium of
ammonia,
3H2(g) + N2(g) --
kf -- > < -- kr -- 2NH3(g)
Dng = 2 - (3+1)
= 2 - 4 = -2
∴ Kp = Kc (RT)-2
Kp = Kc 2
(RT)
and Kp< Kc
2 Dependance of dissociation constant with formation
equilibrium constant
In a formation equilibrium reaction, the reactants and
products are written at the LHS and RHS
of the equilibrium sign respectively. For the same reaction, the dissociation
equilibrium consists of the products in the place of reactants and reactants in the place of products being written at the
LHS and RHS of the equilibrium sign
respectively.
In such cases, the equilibrium constant of the dissociation equilibrium reaction which is also known as the dissociation constant, is found to be the reciprocal value of the equilibrium constant for the formation equilibrium reaction. For example, consider the formation equilibrium reaction of SO3, from SO2 and O2 gases,
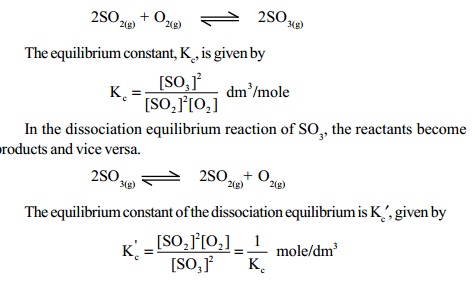
Kc′ is considered as the dissociation constant of SO3 gas. Usually, the equilibrium constant of the dissociation equilibrium is the reciprocal of the equilibrium constant of the formation equilibrium reaction.
3
Applications of law of chemical equilibrium to homogeneous equilibria
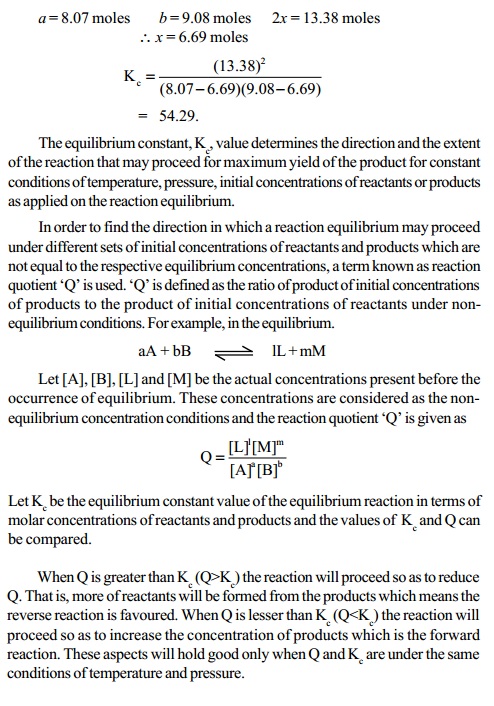
Formation equilibrium of HI
H2(g) + I2(g) -- > < -- 2HI(g)
In this equilibrium reaction, the number of moles of the
products is equal to the number of
moles of the reactants (Dng = 0). Let us
assume 'a' and 'b' moles of H2 and I2 gases being present in 'V' litres of the
reaction vessel. At equilibrium, let
x moles each of H2 and I2 react to form 2x moles of HI. Then, the
equilibrium concentrations in moles litre
of H2, I2 and HI in the reaction mixture will be (a-x)/V, (b-x)/V and 2x/V respectively. Since Dng = 0, Kc = Kp.

Related Topics