Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Abnormal Colligative Properties
Abnormal Colligative Properties
The experimental values of colligative properties in most of the cases
resemble closely to those obtained theoretically by their formula. However, in
some cases experimental values of colligative properties differ widely from
those obtained theoretically. Such experimental values are referred to as
abnormal colligative properties.
The abnormal behaviour of colligative properties has been explained in
terms of dissociation and association of solute molecules.
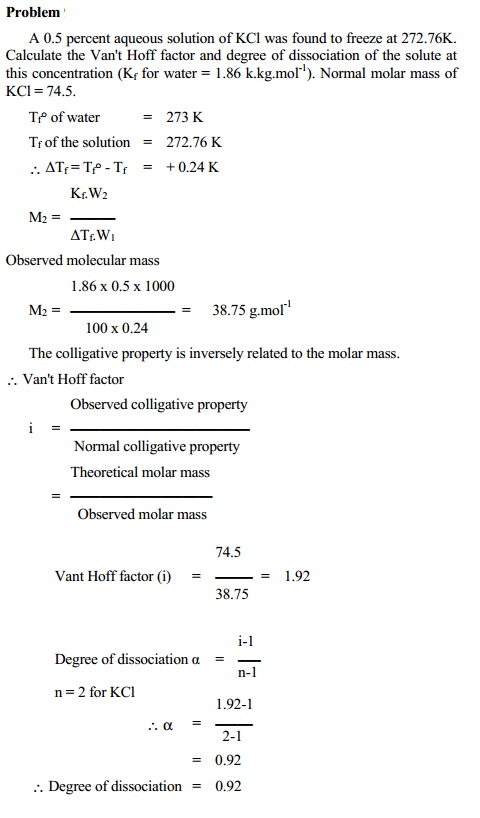
a. Dissociation of solute
molecules
Such solutes which dissociate in solvent (water) i.e. electrolytes, show
an increase in number of particles present in solution. This effect results in
an increase in colligative properties obtained experimentally.
The Van't Hoff factor (i)
i = Experimental colligative property / Normal
colligative property
i > 1 for dissociation. We can calculate the
degree of dissociation (a) using
the equation.
dissociation = i - 1 / n - 1
where `n'
is the total number of particles furnished by one molecule of the solute.
For example, sodium chloride in aqueous solution
exists almost entirely as Na+ and Cl- ions. In such case,
the number of effective particles increases and therefore observed colligative
property is greater than normal colligative property.
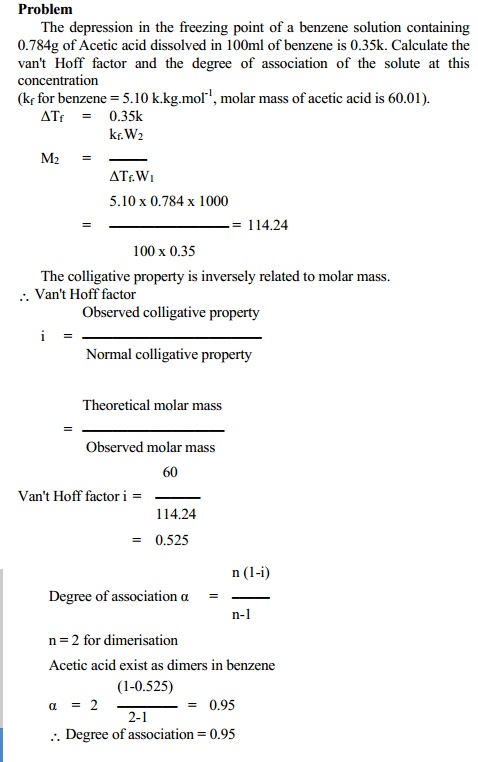
b. Association of the solute molecules
Such solute which associate in a solvent show a
decrease in number of particles present in solution. This effect results in a
decrease in colligative properties obtained experimentally.
Here,
Experimental Colligative Property < Normal
Colligative Property \ Vant Hoff
factor
i = Experimental Colligative Property / Normal
colligative property
i
< 1 for association
Using this, the degree of association 'a' can be
calculated from
a(association) = (1-i)n / (n-1)
where `n' is the number of small molecules that
associate into a single
larger new molecule.
For
example, molecules of acetic acid dimerise in benzene due to intermolecular
hydrogen bonding. In this case, the number of particles is reduced to half its
original value due to dimerisation. In such case, the experimental colligative
property is less than normal colligative property.
2 (CH3COOH)
-- > < -- (CH3COOH)2
Related Topics