Chapter: Modern Analytical Chemistry: Obtaining and Preparing Samples for Analysis
The Importance of Sampling
The Importance of Sampling
When a manufacturer produces a chemical
they wish to list as ACS Reagent
Grade, they must demonstrate that it conforms
to specifications established by the Ameri- can Chemical Society (ACS). For
example, ACS specifications for NaHCO3 require that the concentration of iron be less than
or equal to 0.001% w/w.
To verify that
a production lot meets
this standard, the manufacturer performs
a quantitative analy- sis, reporting the result
on the product’s label. Because
it is impractical to analyze the entire production lot, its properties are estimated from a limited
sampling. Sev- eral samples are collected and analyzed, and the
resulting mean, X–, and standard de- viation, s, are used
to establish a confidence interval for the production lot’s true mean, μ
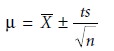
where n is the
number of samples, and t is a statistical factor
whose value is deter-
mined by the number of samples and
the desired confidence level.*
Selecting a sample
introduces a source
of determinate error
that cannot be cor-
rected during the
analysis. If a sample does
not accurately represent the population
from which it is drawn,
then an analysis
that is otherwise carefully conducted will yield inaccurate results. Sampling
errors are introduced whenever we extrapolate
from a sample to its target population. To minimize sampling
errors we must col-
lect the right sample.
Even when collecting the right sample,
indeterminate or random
errors in sam- pling may limit the usefulness of our results.
Equation 7.1 shows
that the width
of a confidence interval is directly proportional to the standard deviation.
The overall standard
deviation for an analysis, so, is determined by ran-
dom errors affecting each step
of the analysis. For convenience, we di-
vide the analysis into two steps. Random
errors introduced when collect-
ing samples are characterized by a standard
deviation for sampling, ss. The standard
deviation for the analytical method,
sm, accounts
for ran- dom errors
introduced when executing the method’s procedure. The re- lationship among
so, ss, and
sm is given by a propagation of random
error
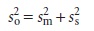
Equation
7.2 shows that an analysis’ overall variance may be lim-
ited by either
the analytical method or sample collection. Unfortu-
nately, analysts often attempt to minimize overall variance by im- proving
only the method’s precision. This is futile, however, if the standard
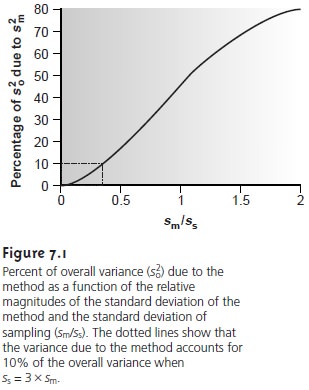
deviation for sampling is more than three times greater than that for the method. Figure 7.1 shows how the ratio sm/ss affects the percentage
of overall variance attributable to the method. When the method’s
standard deviation is one third of that for sampling, indeterminate method errors explain only 10% of the overall variance.
Attempting to improve the analysis by decreasing sm provides
only a nominal
change in the overall variance.
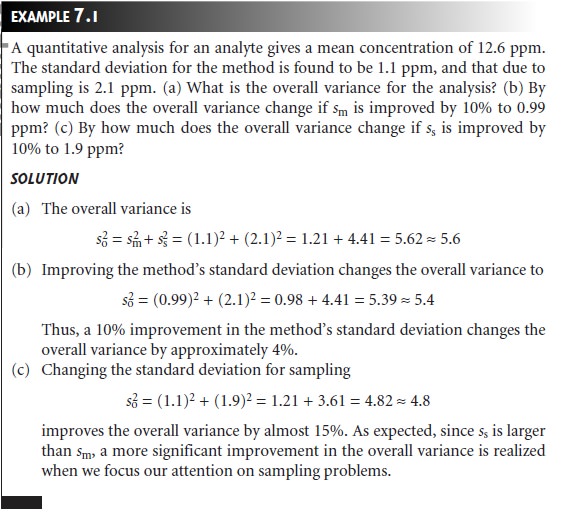
To determine which
step has the greatest effect
on the overall variance, both sm2
and s2 must
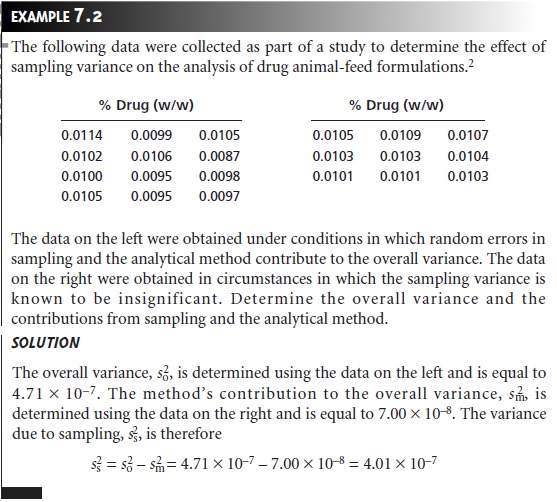
be known. The analysis of replicate samples
can be used to estimate
the overall variance. The variance due to the method is determined by analyzing a stan-
dard sample, for which we may assume
a negligible sampling variance. The variance due to sampling is then determined by difference.
Related Topics