Chapter: Aquaculture Principles and Practices: Nutrition and Feeds
Inorganic fertilizers - Pond fertilization for production of live foods
Inorganic fertilizers
Though considerable experience in the application of inorganic fertilizers has been accumulated by studies made in Eastern and Western Europe and North America, no standardized fertilizer has been evolved similar to the formulae for culture media in controlled live food production discussed. This is only to be expected, in view of the differences in climatic, soil and hydrological conditions that affect fertilizer requirements.
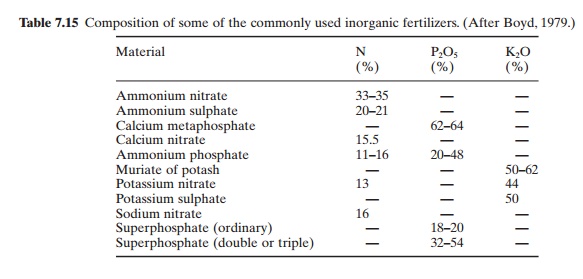
Inorganic fertilizers are in simple inorganic compound form containing at least one of the following primary nutrients: nitrogen, phosphorus and potassium (NPK). They may also include nutrients such as calcium, magnesium and sulphur and trace elements such as copper, zinc, boron, manganese, iron and molybdenum. Generally, aquaculturists use commercially available agricultural fertilizers. The composition of some of the commonly used inorganic fertilizers is given in Table 7.15 (Boyd, 1979).
While NPK fertilizers seem to be favoured in North America, West European aquaculturists lay greater stress on phosphate fertilizers and East Europeans on both nitrogen and phosphorus fertilizers. The main problem with phosphate fertilizers is that phosphorus compounds
are not easily soluble in water and are absorbed by the bottom soil or mud and often converted to insoluble compounds. These are released only when microorganisms change them into assimilable forms, so even though present in the pond, phosphorus is not always available for algal growth. Daily application would be a solution, but the quantities required being small, there are practical difficulties in distributing them evenly in ponds. Mixing the daily require-ment of phosphates with organic manure such as pig manure for application has been found to be beneficial, not only for better distribution, but also because inorganic phosphates are gradually converted into organic phosphate compounds in manures. For this purpose, phosphate fertilizer should be kept mixed with manure for a few days before application. A dose of 0.2–0.5 mg per litre of dissolved P2O5 is considered adequate to maintain a high rate of production of plankton algae. Fish ponds in the former USSR are generally fertilized with 15–20 kg P2O5 per ha. Phosphate fertilizers can have the effect of increasing the nitrogen content of phytoplankton through the fixation of water-soluble nitrogen by nitrogen-fixing bacteria and blue-green algae (Martyshev, 1983).
Nitrogen fertilizers are easily soluble in water and can therefore become readily available for organic production in ponds. A commonly used nitrogen fertilizer is ammonium sulphate, containing 20–21 per cent nitrogen. Urea, which is an organic compound that has to decompose into an inorganic form for absorption by algae, is also used in many aquaculture farms. Liquid ammonia, which is a solution of NH3 in water containing about 20 per cent N by volume, is a cheaper source of nitrogen fertilization in some areas. However, special precautions have to be taken in handling because of its strong odour and possible danger to human health due to inhalation or bodily contact. The usefulness of nitrogen fertilizers has been demonstrated in a number of places. Contrary results reported from Europe have been ascribed to the climatic differences and production pattern in West European fish culture.
A recommended dose of application is 30–40kg ammonium sulphate biweekly or 15–20 kg weekly, together with phosphorus fertilizer. Application of nitrogen fertilizers alone is reported to result in the suppression of nitrogenfixing bacteria under certain conditions. But if there is a vigorous development of phytoplankton, the compounds are reduced at a rapid rate and will not have any suppressing effect on nitrogenfixing bacteria such as Azobacter.
As in the case of nitrogen, there are differences of opinion on the value of potassium fertilizers in ponds. Certain types of soils contain large quantities of potassium, but others such as peaty soils contain little. A large content of potassium in the soil does not ensure its availability in the pond, since most of it is in the form of stable alumosilicate minerals. Exchangeable potassium and water-soluble potassium are present only in small quantities. The rate of potassium fertilizer used varies considerably from 30 to 100 kg/ha, depending on the soil and water conditions.
Calcium fertilizers have a definite role in pond fertilization, but how much of it is a direct contribution to fertility and how much is ameliorative is difficult to assess. Calcium is an essential element of aquatic flora and fauna. It causes precipitation of colloidal humus, reducing its absorption capacity and thereby releasing previously absorbed nutrients into the water. Calcium is added to ponds rich in organic matter and to ponds with acidic soil and water.
Calcium carbonate (CaCO3) and unslaked lime (CaO) are commonly used in ponds. The need for liming to correct acidity in brackish-water swamp soils has been described. According to Martyshev (1983) a dose of 30–5000 kg lime per ha is applied as fertilizer in parts of the former USSR, depending on the conditions in the pond. He quotes norms in other European countries as 1000–2000 kg/ ha in Germany, 200 kg/ha in France, 600–700 kg/ ha in The Netherlands and 500 kg/ha in former Yugoslavia. For a good growth of plankton, the total hardness and total alkalinity of the pond water should not be less than 10 mg/l. Waters with values above 20 mg/l are reported to produce consistently adequate quantities of phytoplankton after inorganic fertilization.
Liming is therefore required when the total alkalinity or hardness is below 20 mg/l. Dark-coloured water containing large quantities of humic substances will also require liming to clear the water and improve light penetration for photosynthesis.
For correction of water quality, much higher doses of lime than mentioned above will be required. Higher acidity requires greater quantities of lime to neutralize it, depending not only on pH but also on the chemical composition of the water, especially the concentration of calcium bicarbonate [Ca(HCO3)2] and its relation with carbon dioxide and carbonates. In Asian brackish-water ponds, with soils of pH value around 5, treatment with 3 tons of agricultural lime (calcium hydroxide Ca(OH)2) per ha are recommended. The lime has to be worked into the pond soil after dewatering. Though agricultural lime will raise soil pH, its effect may not be adequate to maintain the pH of brackish-water ponds because of its low solubility in salt water. Natural carbonates that contain a minimum of about 4 per cent magnesium (such as dolomite, mollusc shells or coral) are more soluble at the pH of sea water and will aid in maintaining optimum alkalinity and pH levels of the pond water. It is therefore desirable for salt-water ponds to have a supply of such types of lime to maintain water quality.
It has been demonstrated that a combination of inorganic fertilizers gives the best results in pond fertilization. Different combinations of the major fertilizers are in common use in many areas. Combinations of the primary fertilizers should be based on the specific requirements of the pond. A dose of 50–60 kg/ha of NPK (16:20:4) fertilizer combination has given satis-factory results in many situations, particularly in fresh waters. When the pond soil has high potassium contents, the potassium can be omitted from the fertilizer.
Studies made in the former USSR seem to show that the addition of certain trace elements promotes plankton production. The application of a fertilizer consisting of 10kg/ha cobalt, 1200kg/ha ammonium nitrate and 200 kg/ha superphosphate results in a marked increase in the biomass of plankton and benthos (Martyshev, 1983). It has also been shown that cobalt is absorbed and retained by pond silt for a long period of time and utilized in the biological cycle. Among the other trace elements studied, boron and molybdenum have proved to be beneficial. The addition of these at the rate of 0.07 g/m3 and 0.01 g/m3 respectively is reported to result in an increase of zooplank-ton biomass of about 150–200 per cent.
However, the accumulation of these trace elements in the species of fish or shellfish cultured has not been studied, and so their use in commercial aquaculture is not widely accepted.
The common practice is to apply about 50 per cent of the total fertilizer requirements initially in preparation for the release of the stock, so that a standing crop of the desired food organisms will develop. Where liming is required to improve the soil or water conditions, this has to be done two to three weeks before the application of nitrogen and phosphorus fertilizers.
As chemical fertilizers are rapidly utilized and large doses inhibit bacterial growth, it is preferable to apply them after the ponds are filled with water. Fertilizers applied on the pond bottom may also be adsorbed by the bottom soil and used by rooted plants rather than plankton. In temperate climates, the initial application is usually in spring, when temperature conditions are optimal for rapid growth of micro-organisms and when major rearing activities begin. In tropical climates, where growth occurs throughout the year, the main consideration is the commencement of a new crop of the aquaculture species. After the required bloom of micro-organisms has developed in the ponds and the stock of young ones has been introduced for rearing, further fertilization is intended only to maintain the density of the required food organisms.
The method of application of the fertilizer is important. Distribution should be as even as possible, to ensure full utilization and prevent loss by precipitation or release into the atmosphere that may happen when it is applied in a limited area. One method of application recommended is dissolving the fertilizer in a suitable container and distributing the solution over the pond surface from a boat. Another method is to apply the fertilizer in a dry powdered form, with a suitable blower. Hepher and Pruginin (1981) described a method of using currents caused by winds in ponds to distribute fertilizers. The fertilizer is deposited on the windward side of the pond at a spot 2–3 m away from the bank, preferably when the wind is blowing.The dissolving fertilizer is carried away by the current generated by the wind and distributed throughout the pond.
In Southeast Asian brackish-water ponds, fertilization is performed to develop the algal complexes known as ‘lab lab’ and ‘lumut’. The ‘lab lab’ complex predominantly consists of benthic blue-green algae (Myxophyceae) and diatoms (Bacillariophyceae), whereas the ‘lumut’ complex is composed primarily of fila-mentous green algae and associated forms of life. Though organic manures are preferred for growing such algal complexes, inorganic fertilizers can also be used either independently or mixed with organics. The dosage recommended is 50–100kg of 18:46:0 (NPK) or 100–150 kg of 16:20:0 (NPK) fertilizer, depending on soil conditions. These are applied on the dry pond bottom and 3–5 cm of water let into the pond soon after treatment. After one week, the same amount of fertilizer is applied again and the water level is raised to 10–15 cm. Fertilization is repeated after two weeks and the water level raised to 20–25 cm. The level of water is topped up to make up for the loss by evaporation. The best growth of ‘lab lab’ occurs in water salinities around 25 ppt or higher. The maximum water level is about 40 cm.
‘Lumut’ grows best in low to medium salinity ranges, above 25 ppt and at water depths of 40–60 cm. Soft mud bottoms with a pH of 6.8–7.5 are considered most favourable for its growth. In cases where the pH is below 6.5, liming should be done in such a way as to incorporate it in the soil. The pond bottom is dried for about three days, after which sufficient water is let in to wet the soil. The pond bottom is then seeded with the desired species of green algae (Oscillatoria, Lyngbia, Phormidium, Spirulina, Microcoleus, etc.) by sticking young filaments into the mud. The pond is then filled to a depth of 20 cm. Three to seven days after planting, the pond is fertilized with 16:20:0 (NPK) fertilizer at the rate of 18–20 g/m3 water. The fertilizer can be broadcast over the pond or dissolved into the water from a submerged platform (about 10 cm below the surface). After a week, the water level is raised to 40 cm. Starting with the second week, a weekly application of the fertilizer at the rate of 9–10 g/m3 water is recommended for the duration of the culture.
The above fertilizer treatment and water management result in the production not only of green algae, but also of a series of associated organisms including bacteria, protozoans, diatoms, nematodes, small crustaceans, etc., which contribute to the live food resources of the pond.
Related Topics