Chapter: Modern Analytical Chemistry: Obtaining and Preparing Samples for Analysis
Implementing the Sampling Plan: Solutions
Solutions
Typical examples of liquid samples
include those drawn
from containers of com-
mercial solvents; beverages, such as milk or fruit juice; natural
waters, including from lakes,
streams, seawater, and
rain; bodily fluids,
such as blood
and urine; and, suspensions, such as those
found in many oral medications.
Sample Collection
Homogeneous solutions are easily sampled by siphoning, de-
canting, or by using a pipet or syringe. Unfortunately, few solutions are
truly homo- geneous. When the material
to be sampled is of manageable size,
manual shaking is often
sufficient to ensure
homogeneity. Samples may then be collected with a pipet, a
syringe, or a bottle. The majority of solutions, however,
cannot be sampled
in this manner. To minimize the effect of heterogeneity, the method for collecting the gross sample must be adapted to the material
being sampled.
The environmental sampling
of waters and wastewaters provides
a good illus- tration of many
of the methods
used to sample
solutions. The chemical composi- tion of surface
waters, such as streams, rivers,
lakes, estuaries, and oceans, is influ-
enced by flow rate and depth. Rapidly
flowing shallow streams
and rivers, and
shallow (<5 m) lakes are usually well mixed and show little stratification with depth. Grab
samples are conveniently collected by submerging a capped bottle
below the surface
and removing the cap. The air–water interface, which may be en-
riched with heavy metals9 or
contaminated with oil, is avoided
when collecting the sample. After the sample bottle is filled, the cap is replaced and the bottle removed.
Slowly moving streams and rivers,
lakes deeper than 5 m, estuaries, and oceans may show
substantial stratification. Grab
samples from near
the surface can
be collected as described earlier, whereas samples
at greater depths
are collected with
a weighted sample bottle
that is lowered
to the desired depth. Once it has reached the desired
depth, the sample bottle is opened, allowed
to fill, and closed before
retrieving. Grab samples can be analyzed
individually, giving information about changes in the
analyte’s concentration with depth. Alternatively, the grab samples
may be pooled to form a composite sample.
Wells used for collecting groundwater samples must be purged before
the sam- ple is collected, since
the chemical composition of water in the well-casing and in the adjacent
matrix may be significantly different from that of the surrounding groundwater. These
differences may result from contaminants introduced when drilling the well, or differences in the groundwater’s redox potential when exposed
to atmospheric oxygen.
In general, wells
are purged by pumping out a volume
of water equivalent to several well-casing volumes, or until the water’s
temperature, pH, or specific
conductance are constant. Samples collected from municipal water supplies must also be purged since the chemical
composition of water left standing in pipes may differ
significantly from the treated water
supply. Samples are collected
at faucets after flushing the pipes for 2–3 min.
Samples from municipal wastewater treatment plants
and samples of industrial
discharges often are collected as 24-h composites. Samples are obtained
using an automatic sampler
that periodically removes
individual grab samples.
The volume of each sample increment and the frequency of sampling may be constant
or may vary in response to changes in flow rate.
Sample containers for collecting solutions are made from glass or plastic. Con- tainers made from Kimax
or Pyrex brand
borosilicate glass have
the advantage of being sterilizable, easy to clean, and inert to all solutions
except those that are strongly alkaline. The disadvantages of glass containers are cost, weight,
and the likelihood of breakage. Plastic
containers are made from a variety of polymers, in- cluding polyethylene, polypropylene, polycarbonate, polyvinyl chloride, and Teflon
(polytetrafluoroethylene). Plastic containers are lightweight, durable, and,
except for those manufactured from Teflon, inexpensive. In most cases
glass or plastic
bot- tles may be used, although
polyethylene bottles are generally preferred because of their lower cost. Glass containers are always used when collecting samples for the analysis of pesticides, oil
and grease, and
organics because these
species often inter- act with plastic surfaces. Since glass surfaces easily adsorb metal
ions, plastic bottles are preferred when collecting samples for the analysis of trace metals.
In most cases
the sample bottle
has a wide mouth, making
it easy to fill and
re- move the sample.
A narrow-mouth sample
bottle is used when exposing
the sample to the container cap or to the outside
environment is undesirable. Unless exposure to plastic
is a problem, caps for sample bottles
are manufactured from polyethylene.
When polyethylene must
be avoided, the
container cap includes an inert interior liner of neoprene or Teflon.
Sample Preservation
Once removed from its target population, a liquid sample’s chemical composition may change as a result of chemical, biological, or physical processes.
Following its collection, samples are preserved by controlling the solution’s pH and temperature, limiting
its exposure to light or to the atmosphere, or by
adding a chemical preservative. After
preserving, samples may be safely
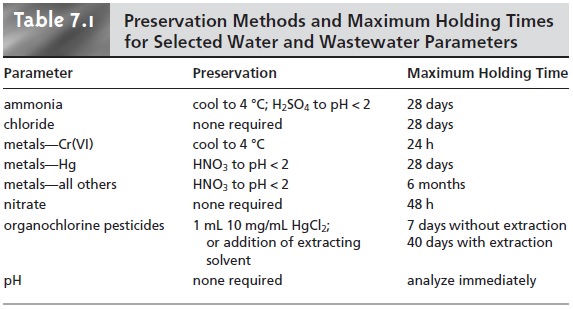
stored for later analysis. The maximum holding
time between preservation and analysis de- pends on the analyte’s stability and the effectiveness of sample preservation. Table 7.1 provides a list of sample preservation methods and maximum
holding times for sev-
eral analytes of importance in the analysis of water and
wastewater.
Sample Preparation
Most analytical methods
can be applied to analytes
in a liquid or solution state.
For this reason
a gross sample
of a liquid or solution
does not need additional processing to bring
it into a more suitable
form for analysis.
Related Topics