Solved Example Problems - Evaluate Yourself - Chemistry: Physical and Chemical Equilibrium | 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Evaluate Yourself - Chemistry: Physical and Chemical Equilibrium
Physical and Chemical Equilibrium
Evaluate Yourself
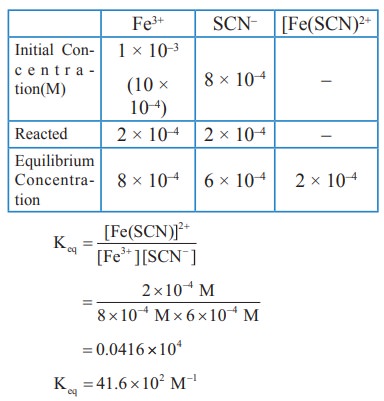
1) Consider the following reaction
Fe3+(aq) + SCN–(aq) ⇌ [Fe(SCN)]2+(aq)
A solution is made with initial Fe3+, SCN- concentration of 1 x 10-3M and 8 x 10-4 M respectively. At equilibrium [Fe(SCN)]2+ concentration is 2 x 10-4M. Calculate the value of equilibrium constant.
Solution:
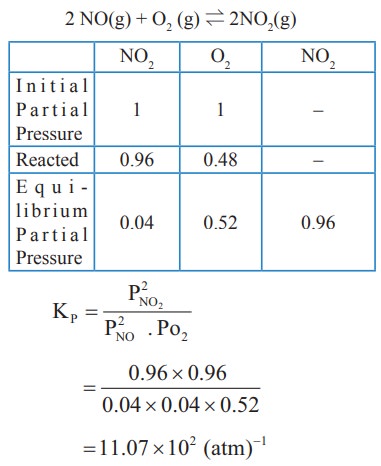
2) The atmospheric oxidation of NO
2NO(g) + O2(g) ⇌ 2NO2(g)
was studied with initial pressure of 1 atm of NO and 1 atm of O2. At equilibrium, partial pressure of oxygen is 0.52 atm calculate Kp of the reaction.
Solution:
3) The following water gas shift reaction is an important industrial process for the production of hydrogen gas.
CO(g) + H2O(g) ⇌ CO2(g) + H2(g)
At a given temperature Kp = 2.7. If 0.13 mol of CO, 0.56 mol of water, 0.78 mol of CO2 and and 0.28 mol of H2 are introduced into a 2 L flask, and find out in which direction must the reaction proceed to reach equilibrium
Solution:
CO(g) + H2O(g) ⇌ CO2 (g) + H2(g)
Given KP = 2.7
[CO] = 0.13, [H2O] = 0.56
[CO2] = 0.78 ; [H2] = 0.28
V = 2L
KP = KC (RT)
2.7 = KC (RT)º
KC = 2.7
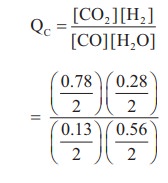
Q = 3
Q > KC, Hence the reaction proceed in the reverse direction.
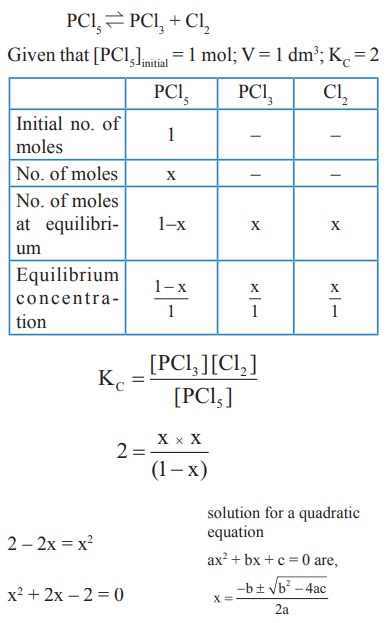
4) 1 mol of PCl5, kept in a closed container of volume 1 dm3 and was allowed to attain equilibrium at 423 K. Calculate the equilibrium composition of reaction mixture. (The Kc value for PCl5 dissociation at 423 K is 2)
Solution:
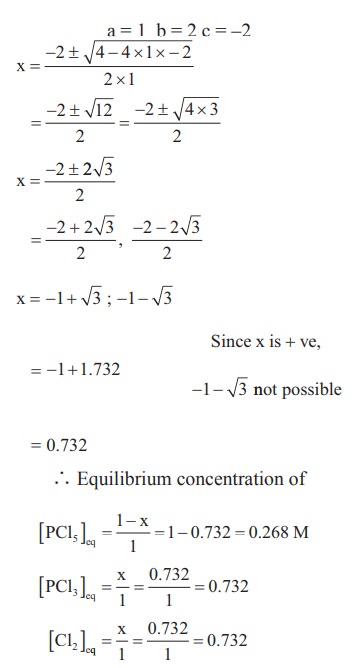
5) The equilibrium constant for the following reaction is 0.15 at 298 K and 1 atm pressure.
N2O4(g) ⇌ 2NO2(g);
ΔHºf = 57.32 KJmol-1
The reaction conditions are altered as follows.
a) The reaction temperature is altered to 100o C keeping the pressure at 1 atm,
Calculate the equilibrium constant.
Solution:
N2O4(g) ⇌ 2NO2(g)
T1 = 298 K
KP1 = 0.15
T2 = 100º C = 100 + 273 = 373 K ;
KP2= ?
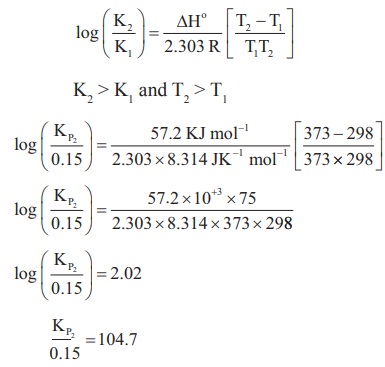
KP2=104.7x0.15
KP2=15.705
Related Topics