Chapter: 11th Chemistry : UNIT 8 : Physical and Chemical Equilibrium
Equilibrium involving dissolution of solids or gases in liquids
Equilibrium involving dissolution of solids or gases in liquids
Solid in liquids
When you add sugar to water at a particular temperature, it dissolves to form sugar solution. If you continue to add much sugar, you will reach a stage at which the added sugar remains as solid and the resulting solution is called a saturated solution. Here, as in the previous cases a dynamic equilibrium is established between the solute molecules in the solid phase and in the solution phase.
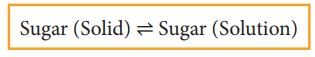
In this process
Rate of dissolution of solute = Rate of crystallisation of solute
Gas in liquids
When a gas dissolves in a liquid under a given pressure, there will be an equilibrium between gas molecules in the gaseous state and those dissolved in the liquid.
Example:
In carbonated beverages the following equilibrium exists.
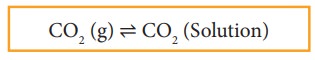
Henry’s law is used to explain such gas-solution equilibrium processes.
Related Topics