Chapter: 11th 12th std standard Class Organic Inorganic Physical Chemistry Higher secondary school College Notes
Electronic configuration and periodic table
Electronic configuration and periodic table1
There is a close connection between the electronic configuration of the
elements and the long form of the Periodic Table. We have already learnt that
an electron in an atom is characterized by a set of four quantum numbers and
the principal quantum number (n) defines the main energy level known as the Shell. The electronic configuration of
elements can be best studied in terms of variations in periods and groups of
the periodic table.
(a) Electronic Configuration in periods
Each successive period in the periodic table is associated with the
filling up of the next higher principal energy level (n=1, n=2,etc.). It can be
readily seen that the number of elements in each period is twice the number of
atomic orbitals available in the energy level that is being filled. The first
period starts with the filling of the lowest level (1s) and has thus the two
elements-hydrogen (1s1) and helium (1s2) when the first
shell (K) is completed. The second period starts with lithium and the third
electron enters the 2s orbital. The next element, beryllium has four electrons
and has the electronic configuration 1s22s2. Starting
from the next element boron, the 2p orbitals are filled with electrons when the
L shell is completed at neon (2s22p6). Thus there are 8
elements in the second period. The third period (n=3) begins at sodium, and the
added electron enters a 3s orbital. Successive filling of 3s and 3p orbitals
gives rise to the third period of 8 elements from sodium to argon.
The fourth
period (n=4) starts at potassium with the filling up of 4s orbital. Now you may
note that before the 4p orbital is filled, filling up of 3d orbitals becomes
energetically favourable and we come across the so-called 3d Transition Series
of elements. The fourth period ends at krypton with the filling up of the 4p
orbitals. Altogether we have 18 elements in this fourth period. The fifth
period (n=5) beginning with rubidium is similar to the fourth period and
contains the 4d transition series starting at yttrium (Z=39). This period ends
at xenon with filling up of the 5p orbitals. The sixth period (n=6) contains 32
elements and successive electrons enter 6s, 4f, 5d and 6p orbitals, in that
order. Filling up of the 4f orbitals begins with cerium (Z=58) and ends at
lutetium (Z=71) to give the 4f-inner transition series, which is called the Lanthanoid Series. The seventh period
(n=7) is similar to the sixth period with the successive filling up of the 7s,
5f, 6d and 7p orbitals and includes most of the man-made radioactive elements.
This period will end at the element with atomic number 118 which would belong
to the noble gas family. Filling up of the 5f orbitals after actinium (Z=89)
gives the 5f-inner transition series known as the Actinoid Series. The 4f- and 5f- transition series of elements are
placed separately in the periodic table to maintain its structure and to
preserve the principle of classification by keeping elements with similar
properties in a single column.
(b) Groupwise/electronic configuration
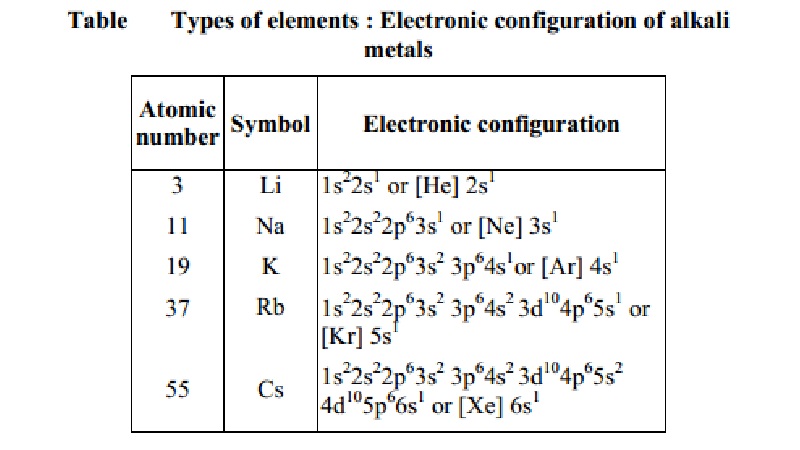
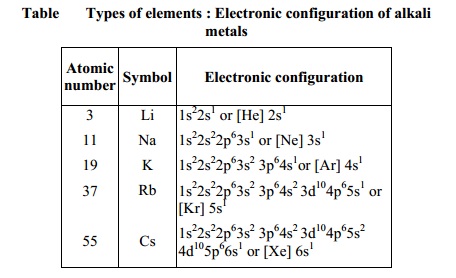
Elements in the same vertical column or group have similar electronic
configurations, have the same number of electrons in the outer orbitals, and
similar properties. Group 1 (the alkali metals) is an example.
Thus it can be seen that the properties of an
element have periodic dependence upon the atomic number and not on relative
atomic mass (Table).
Types of elements: s-, p-,d-, f-
Blocks
The aufbau
principle and the electronic configuration of atoms provide a theoretical
foundation for the periodic classification. The elements in a vertical column
of the periodic table constitute a group or family and exhibit similar chemical
behaviour. Strictly, helium belongs to the s-block but its positioning in the
p-block along with other group 18 elements is justified because it has a
completely filled valence shell (1s2) and as a result, exhibits
properties characteristic of other noble gases. The other exception is
hydrogen. It has a lone s- electron and hence can be placed in group 1 (alkali
metals). It can also gain an electron to achieve a noble gas arrangement and
hence it can behave similar to a group 17 (halogen family) elements. Because it
is a special case, we shall place hydrogen separately at the top of the
Periodic Table. We will briefly discuss the salient features of the four types
of elements marked in the periodic table.
s-Block Elements
The elements of group 1 (alkali metals) and
group 2 (alkaline earth metals) which have ns1 and ns2
outermost electronic configuration belong to the s-block elements. They
are all reactive metals with low ionization enthalpies. They lose the outermost
electron(s) readily to form 1+ (in the case of alkali metal) or 2+ ions (in the
case of alkaline earth metals). The metallic character and the reactivity
increase as we go down the group. The compounds of the s-block elements, with the exception of those of beryllium are
predominantly ionic.
p-Block Elements
The p-Block Elements comprise those belonging to groups 13 to 18
and together with the s-block elements are called the Representative Elements or
Main Group Elements. The outermost electronic configuration varies from ns2np1 to ns2np6
in each period. Each period ends in a noble gas with a closed shell ns2np6
configuration. All the orbitals in the valence shell of the noble gases are completely filled by
electrons and it is very difficult to alter this stable arrangement by the
addition or removal of electrons. The noble gases thus exhibit very low
chemical reactivity. Preceding the noble gas family are two chemically
important groups of nonmetals. They are the halogens (groups 17) and chalcogens
(group 16). These two groups of elements have higher negative electron gain enthalpies and readily add one or two
electrons respectively to attain the stable noble gas configuration. The
nonmetallic character increases as we move from left to right across a period
and metallic character increases as we go down the group.
The d-block
Elements (Transition Elements)
These are the elements of group 3 to 12 in the
center of the periodic table. These elements are characterized by filling of
inner d orbitals by electrons and are therefore referred to as d-Block
Elements. These elements have the outer electronic configuration (n-1) d1-10
ns1-2. They are all metals. They mostly form colored ions and
exhibit variable valency. However, Zn, Cd and Hg, which have the (n-1)d10
ns2 electronic configuration, do not show most of the properties of
transition elements in a way, transition metals form a bridge between the
chemically active metals of s-block elements and less active metals of groups
13 and 14 and thus take their familiar name 'transition elements'
The f-Block
Elements (Inner-Transition elements)
The two rows of elements at the bottom of the periodic table, called the
Lanthanoids 58Ce-71Lu
and Actinoids. 90Th-103Lr
are characterized by the outer electronic configuration (n-2) f1-14 (n-1) d0-10ns2.
The last electron added to each element is an f-electron. These two series of
elements are hence called the inner transition elements (f-Block Elements).
They are all metals within each series, the properties of the elements are
quite similar. The chemistry of the early actinoids is more complicated than
the corresponding lanthanoids, due to the large number of oxidation states
possible for these actinoid elements. Actinoid elements are radioactive. Many
of the actinoid elements have been made only in nanogram quantities or less by
nuclear reactions and their chemistry is not fully studied. The elements coming
after uranium are called transuranium
elements.
Example 1
The elements Z=117 and 120 have not yet been discovered. In which family
/ group would you place these elements and also give the electronic
configuration in each case.
Solution
We see from the periodic table that element with Z=117, would belong to
the halogen family (group 17) and the electronic configuration would be. [Rn]
4f14 5d10 7s2 7p5. the element with
Z=120, will be placed in group 2 (alkaline earth metals), and will have the
electronic configuration [Uuo]8s2.
In addition to displaying the classification of elements into s-, p-,
d-, and f-blocks, the periodic table shows another broad classification of
elements based on their properties. The elements can be divided into Metals and Non-metals. Metals comprise more than 75% of all known elements and appear on the left side
of the Periodic Table. Metals are usually solids at room temperature (Mercury
is an exception); they have high melting and boiling points. They are good
conductors of heat and electricity. They are malleable (can be flattened into
thin sheets by hammering) and ductile (can be drawn into wires). In contrast
non-metals are located at the top right hand side of the Periodic Table.
Non-metals are usually solids or gases at room temperature with low melting and
boiling points. They are poor conductors of heat and electricity. Most
non-metallic solids are brittle and are neither malleable nor ductile. The
elements become more metallic as we go down a group; the non-metallic character
increases as one goes from left to right across the Periodic Table. The change
form metallic to non-metallic character is not abrupt as shown by the thick
zig-zag line in the periodic table. The elements (e.g. germanium, silicon,
arsenic, antimony and tellurium) bordering this line and running diagonally
across the Periodic Table show properties that are characteristic of both
metals and non-metals. These elements are called Semi Metals or Metalloids.
Example 2
Arrange the following elements in the increasing
order of metallic character: Si, Be, Mg, Na, P.
Solution
Metallic character increases down a group and
decreases along a period as we move from left to right. Hence the order of
increasing metallic character is, P<Si<Be<Mg<Na.
Related Topics