Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
Work and Heat
WORK AND HEAT
The different thermodynamic systems and their characteristics were
discussed. To undergo a change of state, the system has to interact with its surroundings.
Work and heat transfers across the boundaries cause these changes.
Work as Defined in Mechanics
work
is done when the point of application of a force moves in the direction of the
force. The product of the force and the distance
moved in the direction of the force is equal to the amount of the work done.
This simple definition
of work confines only to the area of mechanics and can not be extended to the
more complex problems in thermodynamics. Hence a new definition should be
introduced to cover mechanical as well as the other forms of work.
The Thermodynamic Definition of Work
Positive work is
done by a system, during a given process, when sole effect external to the
system could be reduced to the lifting of a mass.
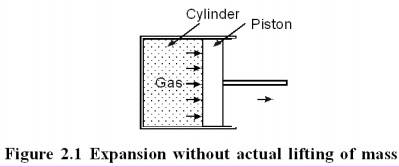
Consider
a gas expanding in a piston cylinder arrangement as given in Figure 2.1. Here
no mass is actually lifted against gravity. But if the existing surroundings is
fitted with an arrangement as given in the Figure 2.2, there is a possibility
of lifting the mass. Hence work is said to be done by the system.
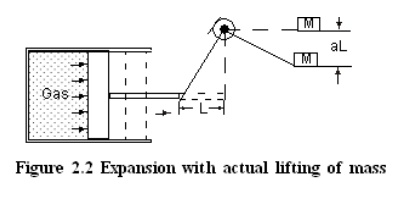
While
exploring the possibility of lifting a mass the effects that are external to
the system alone must be taken into account. For example, a lift with a person
and a suitcase is considered as a system. If the person lifts the suitcase, it
should not be taken into account, because this event occurs within the system.
Related Topics