Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
Sensible and Latent Heat
Sensible and Latent Heat
It is known that a
substance can exists in three phases namely solid, liquid and gas. When a
substance is heated or cooled temperature of the substance increases or decreases
respectively unless there is any phase change. Quantity of heat added or
removed to change the temperature by unit degree is known as specific heat. For
solids and liquids same quantity of heat is required to cause unit degree rise
for both constant pressure heating as well as constant volume heating as they
are incompressible. But for gases there is appreciable difference in the
quantity of heat required to cause unit difference in temperature between
constant volume and constant pressure processes. Accordingly, they are known as
specific heat at constant volume (CV) and specific heat at constant
pressure (CP). Thus to increase the
temperature
of m kg of the given substance by DT
degree, amount of heat required is given by

Q
=mCvDT
at Constant Volume ...(2.5)
Q1
=mCPDT at Constant Pressure …(2.6)
If a certain single
component system is undergoing phase change at constant pressure, temperature
of the system remains constant during heating or cooling. Quantity of heat
removed or added to cause the change of phase of unit mass of the substance is
known as latent heat. For example latent heat of fusion of water is the amount
of heat to be removed to solidify 1 kg of water into 1 kg of ice at a given
temperature.
Let us consider a
process of converting 1 kg of ice at -30°C to system to steam at 250°C at atmospheric pressure. We know that ice melts at
0°C and water evaporates at 100°C at atmospheric pressure.
For
a constant rate of heating, if temperature at different instants are plotted we
will get a graph as shown in Figure 2.9.
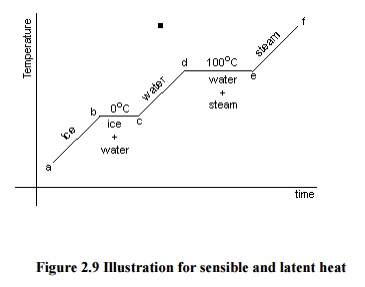
Figure
2.9 Illustration for sensible and latent heat
The
total heat required can be obtained as follows:
Q
= Qab + Qbc + Qcd + Qde + Qef ...(2.7)
Qab = mCice (tb -
tc) ...(2.8)
Qbc = Latent
heat of melting of ice at 0oC
Qcd = mCwater (td
- tc) ...(2.9)
Qde = Latent heat of evaporation of water at 100oC
Qef = mCPSteam (tf
- te) ...(2.10)
Where
Cice =Specific heat of ice
Cwater
= Specific heat of water
CPSteam
=Specific heat of steam at constant
pressure
Reversible Adiabatic Process
A
reversible process during which, the system and the surroundings do not
exchange any heat across the boundary is known as reversible adiabatic process.
For such a process, pressure and volume variation is governed by the law :
pV
=constant . ..(2.11)
Where
Cp
is the specific heat at constant pressure
CV
is the specific heat at constant volume
Detailed
discussion on these specific heats is presented in the next chapter.
A wall which does not
permit the heat flow across it is known as adiabatic wall, whereas the wall
that permits the heat is known as diathermic wall. In an adiabatic process the
only possible energy interaction across the boundary of the system is work transfer
to or from the system.
Displacement
work involved in a reversible adiabatic process can be expressed as
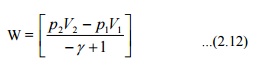
Comparison between work and heat
l Both heat and work are boundary phenomena,
that is, they occur only at the boundary.
l The
interaction due to the temperature difference is heat and all other
interactions are to be taken as work.
l Both
work and heat are path functions, that is, they are inexact differentials.
Related Topics