Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
The Steady-state Flow Process
The Steady-state Flow Process
When
a flow process is satisfying the following conditions, it is known as a steady
flow process.
1. The
mass and energy content of the control volume remains constant with time.
2. The
state and energy of the fluid at inlet, at the exit and at every point within
the control volume are time independent.
3. The
rate of energy transfer in the form of work and heat across the control surface
is constant with time.
Therefore
for a steady flow process

This
equation is commonly known as steady flow energy equation (SFEE).
Application of SFEE
SFEE governs the
working of a large number of components used in many engineering practices. In
this section a brief analysis of such components working under steady flow
conditions are given and the respective governing equations are obtained.
Turbines
Turbines
are devices used in hydraulic, steam and gas turbine power plants. As the fluid
passes through the turbine,
work is done on the blades of the turbine which are attached to a shaft. Due to
the work given to the blades, the turbine shaft rotates producing work.
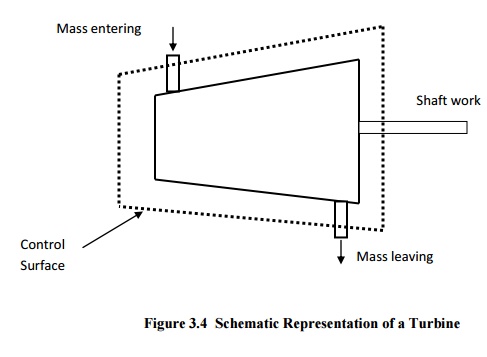
General Assumptions
1.
Changes in kinetic energy of the fluid
are negligible
2.
Changes in potential energy of the fluid
are negligible.
[Q-W
]=m[(h2 -h1
)]
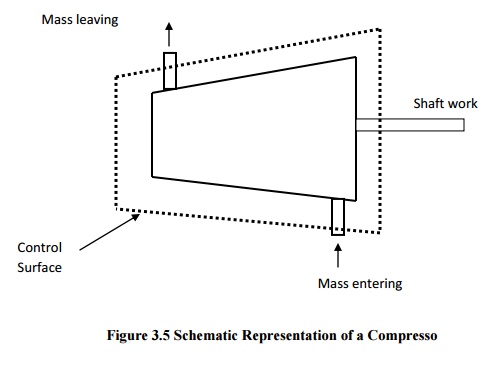
Compressors
Compressors
(fans and blowers) are work consuming devices, where a low-pressure fluid is
compressed by utilising mechanical work. Blades attached to the shaft of the
turbine imparts kinetic energy to the fluid which is later converted into
pressure energy.
General Assumptions
1.
Changes in the kinetic energy of the
fluid are negligible
2.
Changes in the potential energy of the
fluid are negligible
Governing Equation
Applying
the above equations SFEE becomes
[Q-W
]=m[(h2 -h1
)]
Pumps
Similar
to compressors pumps are also work consuming devices. But pumps handle
incompressible fluids, whereas compressors deal with compressible fluids.
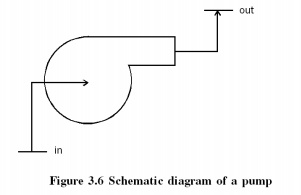
General Assumptions
1. No
heat energy is gained or lost by the fluids;
2. Changes
in kinetic energy of the fluid are negligible.
Governing
Equation
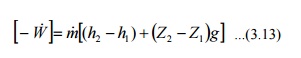
As the fluid passes
through a pump, enthalpy of the fluid increases, (internal energy of the fluid
remains constant) due to the increase in pv (flow energy). Increase in
potential energy of fluid is the most important change found in almost all pump
applications.
Nozzles
Nozzles are devices
which increase the velocity of a fluid at the expense of pressure. A typical
nozzle used for fluid flow at subsonic* speeds is shown in Figure 3.7.
General
Assumptions
1.
In nozzles fluids flow at a speed which
is high enough to neglect heat lost or gained as it crosses the entire length
of the nozzle. Therefore, flow through nozzles can be regarded as adiabatic.
That is =0.
2. There is no shaft or any other form of work transfer
to the fluid or from the fluid; that is
=0.
Changes
in the potential energy of the fluid are negligible.
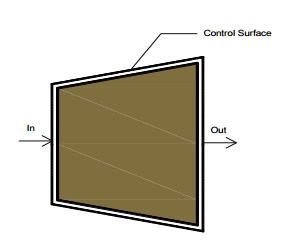
Governing
Equation
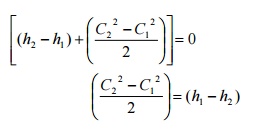
Diffusers
Diffusers
are (reverse of nozzles) devices which increase the pressure of a fluid stream
by reducing its kinetic energy.
General
Assumptions
Similar
to nozzles, the following assumptions hold good for diffusers.
1. Heat lost or gained as it crosses the
entire length of the nozzle. Therefore, flow through nozzles can be regarded as
adiabatic. That is Q = 0
2. There is no shaft or any other form of
work transfer to the fluid or from the fluid; that is = 0.
3. Changes in the potential energy of the
fluid are negligible
Governing
Equation
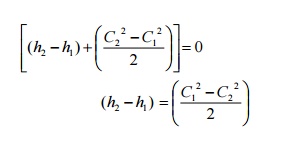
Heat Exchangers
Devices in which heat
is transferred from a hot fluid stream to a cold fluid stream are known as heat
exchangers.
Throttling
A throttling process
occurs when a fluid flowing in a line suddenly encounters a restriction in the
flow passage. It may be
· a
plate with a small hole as shown in Figure 3.10 (a)
· a
valve partially closed as shown in Figure 3.10 (b)
· a
capillary tube which is normally found in a refrigerator as shown in Figure
3.10 (c)
· a porous plug as shown in Figure 3.10 (d)

First
Law for a Cyclic Process
In
a cyclic process the system is taken through a series of processes and finally
returned to its original state. The end state of a cyclic process is identical
with the state of the system at the beginning of the cycle. This is possible if
the energy level at the beginning and end of the cyclic process are also the
same. In other words, the net energy change in a cyclic process is zero.
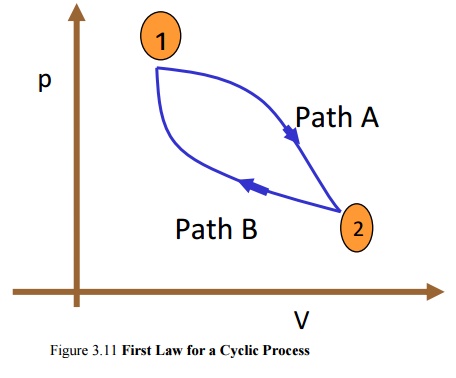
Figure
3.11 First Law for a Cyclic Process
Consider
a system undergoing a cycle consisting of two processes A & B as shown in
Figure 3.11 Net energy change
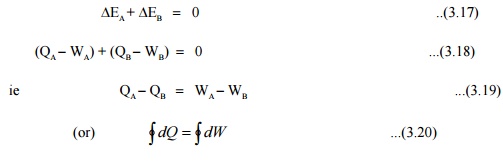
Hence for a
cyclic process algebraic sum of heat tranfers is equal to the algebraic sum of
work transfer.
This was first proved
by Joule, based on the experiments he conducted between 1843 and 1858, that
were the first quantitative analysis of thermodynamic systems.
Energy
is a property of a system
Consider
a system undergoing a process from state1 to state2 along path A as shown in
Figure 3.12. Let the system be taken back to the initial state 1 along two
possible paths B and C. Process A, combined separately with process B and C
forms two possible cycles.
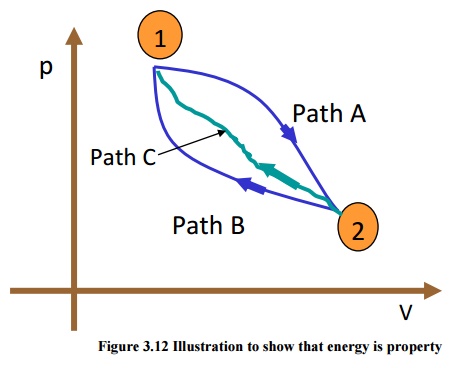
Cycle 1A2B1
QA
+QB =[WA
+WB]
QA
-WA =-[QB
-WB]
E = - DE D ...(3.21)
Cycle 1A2C1
QA
+QC =[WA
+WC]
QA
-WA =-[QC
-WC]
DEA =
- DEC ...(3.22)
From Equation (3.21)
and (3.22) it can be concluded that energy change in path B and path C are
equal and hence energy is a point function depending only on the end states.
It has been already
shown that all the properties are point functions and hence energy is also a
property of the system.
Specific
Heat at Constant Volume and at Constant Pressure
Specific
heat at constant volume of a substance is the amount of heat added to rise the
temperature of unit mass of the given substance by 1 degree at constant volume
From
first law for a stationary closed system undergoing a process
dQ
=pdV + dU or dq =pdv + du
For
a constant volume process
dQ
=dU or dq =du
du
=C dT ...(3.23)
Similarly
specific heat at constant pressure is the quantity of heat added to rise the
temperature of unit mass of the given substance by 1 degree at constant
pressure
where dQ =pdV
+ dU
=pdV + d(H -PV)
dQ = pdV + dH -Vdp -pdV
dQ =dH -Vdp
For a constant pressure process dp =0
Hence dQ =dH
or dq =dh
\
or
dh =CpdT
· The difference
in specific heats Cp -Cv
=R
· The ratio of sp.
heat g =Cp/Cv
· Since h and u
are properties of a system, dh =CpdT
and du=CvdT, for all processes.
Work
Interaction in a Reversible Steady Flow Process
In
a steady flow process the work interaction per unit mass between an open system
and the surroundings can be expressed in
differential form as
dq
- dw - dh + CdC + gdz
dw - dq -
(dh + CdC +gdz)
dq - du + pdv (or) dh - vdp
dw - dh -
vdp - (dh + CdC + gdz)
-
vdp - (CdC + gdz)
For
a stationary
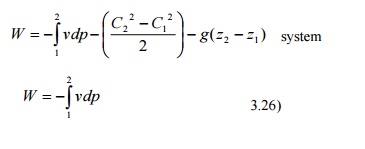
First
law for an open system under unsteady flow conditions
Many
processes of engineering interest involve unsteady flow, where energy and mass
content of the control volume increase or decrease.
Example
for such conditions are:
1) Filling
closed tanks with a gas or liquid.
2) Discharge
from closed vessels.
3) Fluid
flow in reciprocating equipments during an individual cycle.
To
develop a mathematical model for the analysis of such systems the following
assumptions are
made.
instant of time the state is uniform throughout the
entire control volume.
3) The
state of the mass crossing each of the areas of flow on the control surface is
constant with time although the mass flow rates may be time varying.
Unlike
in steady flow system, duration of observation Dt
plays an important role in transient analysis. Let mass of the working fluid
within the control volume before and after the observation be m1 and
m2 respectively. Applying mass balance we get,
(m2
-m1)CV =Smi -Sm0 ...(3.27)
Where ∑mi is the mass entered the control volume during the interval Dt seconds.
∑m0 is the mass left the control volume during the interval Dt seconds.
By
applying energy balance we get,
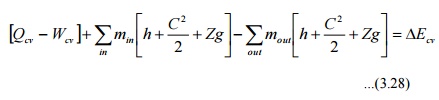
Where
ECV is the change in energy content of the control volume in Dt seconds.
QCV is the heat energy entered into the
control volume in Dt seconds.
WCV is the work energy left the control
volume in Dt seconds.
hi
& h0 are specific enthalpy of the inlet and outlet streams
respectively.
are the kinetic energy of the inlet and
outlet streams respectively.
Zig
& Z0g are the potential energy of inlet and outlet streams
respectively.
Perpetual
Motion Machine – I
An
engine which could provide work transfer continuously without heat transfer is known
as perpetual motion machine of first kind. It is impossible to have such an
engine as it violates first law of thermodynamics.
Related Topics