Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
Processes and Cycles
Processes and Cycles
When a system is taken
from one equilibrium state to another, the change is known as process. The
series of intermediate states through which a system passes during a process is
called the path of the process. If all these intermediate states are
equilibrium states, the process is known as quasi equilibrium or quasi-static
process.
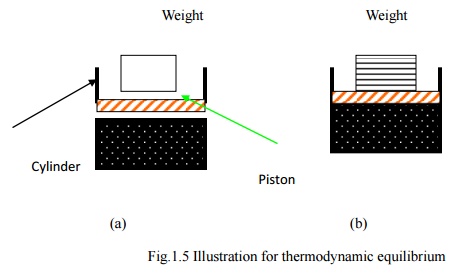
Consider
a certain quantity of gas taken in a frictionless piston cylinder arrangement
as shown in Fig 1.5. The system is in thermodynamic equilibrium so that there
is no unbalanced force acting on piston.
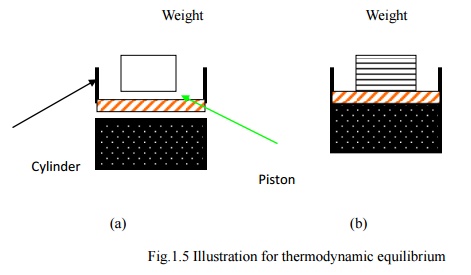
Fig.1.5
Illustration for thermodynamic equilibrium
The moment the weight
is removed from the piston, mechanical equilibrium does not exist and as a
result the piston is moved upward until mechanical equilibrium is restored
again. Therefore the actual process occurs only when equilibrium does not
exist.
As shown in Fig.1.5.a,
if the entire weight on the piston is removed at once, the deviation from the
equilibrium is high and the expansion is rapid. For such a process the
intermediate states are not equilibrium states and hence the process would be
non-quasi-equilibrium.
If the weight is
assumed to be made of a large number of small pieces as shown in Fig.1.5.b and
taken off one by one, the deviation from equilibrium is less. The process could
be considered quasi-equilibrium.
A thermodynamic system
is said to undergo a cycle, if it is taken through a number of processes such
that, the final state of the last process is identical with the initial state
of the first process in all respects. For cycles net change in any property is
zero.
Related Topics