Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
Temperature and Zeroth Law
Temperature and Zeroth Law
Maxwell defined the
temperature of a system as its Thermal state considered with reference to its
ability to communicate heat to other bodies.
When a hot body is
brought into contact with a cold body, the hot body becomes cooler and the cold
body becomes hotter. After sufficient time, the temperature of both the bodies
will be equal. At that point, the two bodies are said to have reached thermal
equilibrium.
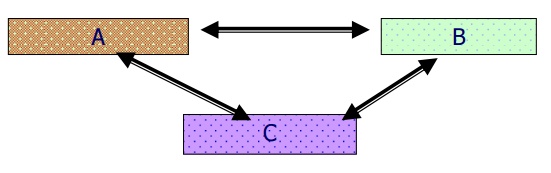
Consider three
bodies A, B and C. If the bodies A and B are in thermal equilibrium with C when
brought into contact separately, they are also in thermal equilibrium with each
other. This concept is known as zeroth law of thermodynamics.
Several properties of materials are
found to be varying with temperature in a predictable way. This variation is
used to measure temperature. In mercury thermometers, expansion of mercury with
temperature is used for temperature measurement.
BASIC CONCEPTS AND DEFINITIONS
Thermodynamics is the
science of energy transfer which deals with the relations among heat, work and
properties of systems.
The name ‘thermodynamics’ is derived from the
Greek words therme, meaning ‘heat’ and dynamis meaning
power. Thus, thermodynamics is basically the study of heat and power.
Application Area of Thermodynamics
Energy transfer is
present in almost all the engineering activities. Hence, the principles of
thermodynamics are playing vital role in designing all the engineering
equipments such as internal combustion engines, rockets, jet engines, thermal
and nuclear power plants, refrigerators etc.
Related Topics