Chapter: Mechanical : Engineering Thermodynamics : Basic Concepts And Definitions
First Law of Thermodynamics
THE FIRST LAW OF
THERMODYNAMICS
Energy interactions
between a system and its surroundings across the boundary in the form of heat
and work have been discussed separately in the previous chapter. So far, no
attempt has been made to relate these interactions between themselves and with
the energy content of the system.
First law of
thermodynamics, often called as law of conservation of energy, relating work,
heat, and energy content of the system will be discussed in detail in this
chapter.
First Law of Thermodynamics
In
its more general form, the first law may be stated as follows
“When energy is
either transferred or transformed, the final total energy present in all forms
must precisely equal the original total energy”.
It is based on the
experimental observations and can not be proved mathematically. All the observations
made so far, confirm the correctness of this law.
First Law of Thermodynamics for a Closed
System Undergoing a Process
First
law can be written for a closed system in an equation form as
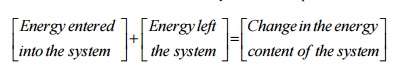
For a system of
constant mass, energy can enter or leave the system only in two forms namely
work and heat.
Let
a closed system of initial energy E1 receives Q units of net heat
and gives out W units of work during a process. If E2 is energy
content at the end of the process as given in Figure 3.1, applying first law we
get
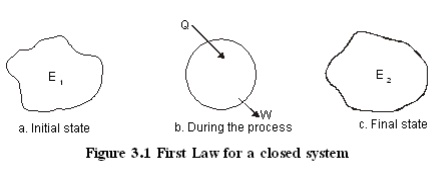
Q -W
=(E2 -E1 )
Where the total energy content
Internal Energy E + Kinetic= energy
+ Potential energy
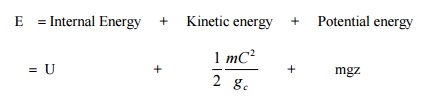
The term internal
energy usually denoted by the letter U is the energy due to such factors as
electron spin and vibrations, molecular motion and chemical bond.
Kinetic
energy term is due to the system movement with a velocity C. For stationary
systems this term will be zero. The term gc is a constant of value 1
in SI unit. It will be dropped here after since SI unit is followed throughout
the book.
Potential energy term
is due to the location of the system in the gravitational field. It remains
constant for a stationary system. The unit of energy in SI is kJ.
The
Thermodynamic Property Enthalpy
Consider a stationary
system of fixed mass undergoing a quasi-equilibrium constant pressure process
Applying
first law
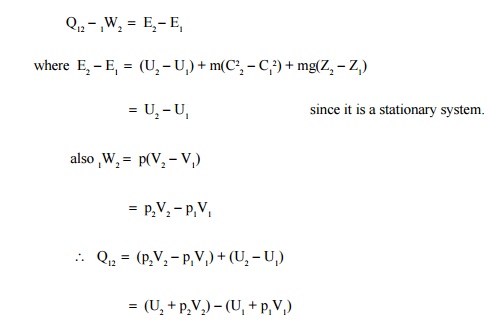
The
terms within brackets are all properties depending on the end states. This combination
of properties may be regarded as a single property known as enthalpy. It is
usually denoted by the letter H.
ie H -U
+ pV
(or) h -u
+ pv
Where h
is specific enthalpy in kJ/kg
u
is specific internal energy in kJ/kg and
v
is specific volume in m3/kg
Flow
Energy
Flow
energy is defined as the energy required to move a mass into the a control
volume against a pressure. Consider a mass of volume V entering into a control
volume as given in the Figure 3.2 against a pressure p.
The
Flow energy =Work done in moving the
mass
=Force ´distance
=pA ´dx
=p ´(Adx)
=pV
Therefore, Enthalpy =Internal energy + Flow energy
First
Law of Thermodynamics for a Control Volume
Mass simultaneously
entering and leaving the system is a very common phenomenon in most of the
engineering applications. Control volume concept is applied to these devices by
assuming suitable control surfaces.
To analyze these
control volume problems, conservation of mass and energy concepts are to be
simultaneously considered.
Energy may cross the
control surface not only in the form of heat and work but also by total energy
associated with the mass crossing the boundaries. Hence apart from kinetic,
potential and internal energies, flow energy should also be taken into account.
Conservation
of mass

Conservation
of energy
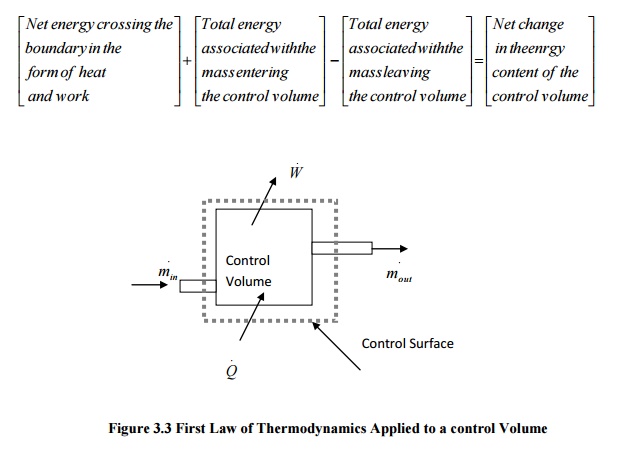
Figure
3.3 First Law of Thermodynamics Applied to a control Volume
As
a rate equation, it becomes
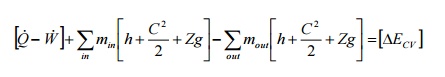
Related Topics